Article contents
Glikinite, Zn3O(SO4)2, a new anhydrous zinc oxysulfate mineral structurally based on OZn4 tetrahedra.
Published online by Cambridge University Press: 30 April 2020
Abstract
A new mineral glikinite, ideally Zn3O(SO4)2, was found in high-temperature exhalative mineral assemblages in the Arsenatnaya fumarole, Second scoria cone of the Great Tolbachik Fissure Eruption (1975–1976), Tolbachik volcano, Kamchatka Peninsula, Russia. Glikinite is associated closely with langbeinite, lammerite-β, bradaczekite, euchlorine, anhydrite, chalcocyanite and tenorite. It is monoclinic, P21/m, a = 7.298(18), b = 6.588(11), c = 7.840(12) Å, β = 117.15(3)°, V = 335.4(11) Å3 and R1 = 0.046. The eight strongest lines of the powder X-ray diffraction pattern [d in Å (I) (hkl)] are: 6.969(56)(00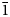 $\bar{1}$), 3.942(52)(101), 3.483(100)(00
$\bar{1}$), 3.942(52)(101), 3.483(100)(00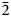 $\bar{2}$), 3.294(49)(020), 2.936(43)(120), 2.534(63)(201), 2.501(63)(20
$\bar{2}$), 3.294(49)(020), 2.936(43)(120), 2.534(63)(201), 2.501(63)(20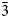 $\bar{3}$) and 2.395(86)(02
$\bar{3}$) and 2.395(86)(02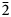 $\bar{2}$). The chemical composition determined by electron-microprobe analysis is (wt.%): ZnO 42.47, CuO 19.50, SO3 39.96, total 101.93. The empirical formula calculated on the basis of O = 9 apfu is Zn2.07Cu0.97S1.98O9 and the simplified formula is Zn3O(SO4)2. Glikinite is a Zn,Cu analogue of synthetic Zn3O(SO4)2. The crystal structure of glikinite is based on OZn4 tetrahedra sharing common corners, thus forming [Zn3O]4+ chains. Sulfate groups interconnect [Zn3O]4+ chains into a 3D framework.
$\bar{2}$). The chemical composition determined by electron-microprobe analysis is (wt.%): ZnO 42.47, CuO 19.50, SO3 39.96, total 101.93. The empirical formula calculated on the basis of O = 9 apfu is Zn2.07Cu0.97S1.98O9 and the simplified formula is Zn3O(SO4)2. Glikinite is a Zn,Cu analogue of synthetic Zn3O(SO4)2. The crystal structure of glikinite is based on OZn4 tetrahedra sharing common corners, thus forming [Zn3O]4+ chains. Sulfate groups interconnect [Zn3O]4+ chains into a 3D framework.
- Type
- Article
- Information
- Copyright
- Copyright © Mineralogical Society of Great Britain and Ireland 2020
Footnotes
Associate Editor: Ian T. Graham
References
- 8
- Cited by




