Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Schütte, Jens
Rahe, Philipp
Tröger, Lutz
Rode, Sebastian
Bechstein, Ralf
Reichling, Michael
and
Kühnle, Angelika
2010.
Clear Signature of the (2 × 1) Reconstruction of Calcite (101̅4).
Langmuir,
Vol. 26,
Issue. 11,
p.
8295.
Pasarín, I. S.
Yang, M.
Bovet, N.
Glyvradal, M.
Nielsen, M. M.
Bohr, J.
Feidenhans’l, R.
and
Stipp, S. L. S.
2012.
Molecular Ordering of Ethanol at the Calcite Surface.
Langmuir,
Vol. 28,
Issue. 5,
p.
2545.
Pasarín, I. S.
Bovet, N.
Glyvradal, M.
Nielsen, M. M.
Bohr, J.
Feidenhans'l, R.
and
Stipp, S. L. S.
2012.
Atomic modifications by synchrotron radiation at the calcite–ethanol interface.
Journal of Synchrotron Radiation,
Vol. 19,
Issue. 4,
p.
530.
Wu, Di
and
Navrotsky, Alexandra
2013.
Small molecule – Silica interactions in porous silica structures.
Geochimica et Cosmochimica Acta,
Vol. 109,
Issue. ,
p.
38.
Imada, Hirotake
Kimura, Kenjiro
and
Onishi, Hiroshi
2013.
Water and 2-Propanol Structured on Calcite (104) Probed by Frequency-Modulation Atomic Force Microscopy.
Langmuir,
Vol. 29,
Issue. 34,
p.
10744.
Okhrimenko, D.V.
Dalby, K.N.
Skovbjerg, L.L.
Bovet, N.
Christensen, J.H.
and
Stipp, S.L.S.
2014.
The surface reactivity of chalk (biogenic calcite) with hydrophilic and hydrophobic functional groups.
Geochimica et Cosmochimica Acta,
Vol. 128,
Issue. ,
p.
212.
Pedersen, N. R.
Hassenkam, T.
Ceccato, M.
Dalby, K. N.
Mogensen, K.
and
Stipp, S. L. S.
2016.
Low Salinity Effect at Pore Scale: Probing Wettability Changes in Middle East Limestone.
Energy & Fuels,
Vol. 30,
Issue. 5,
p.
3768.
Marcano, Maria C.
Kim, Sooyeon
Taylor, Sandra D.
and
Becker, Udo
2019.
Exploring wettability by imaging the adsorption of crude oil, re-dissolved asphaltene, and phenol solutions onto calcite. Implications to sorption mechanisms and molecular structure of surface-active compounds in crude oil.
Chemical Geology,
Vol. 525,
Issue. ,
p.
462.
Marcano, Maria C.
Kim, Sooyeon
and
Becker, Udo
2020.
Surface interaction of crude oil, maltenes, and asphaltenes with calcite: An atomic force microscopy perspective of incipient wettability change.
Applied Geochemistry,
Vol. 113,
Issue. ,
p.
104501.
Ye, Shaoxiong
Feng, Pan
and
Liu, Jiaping
2020.
Concrete Durability and Service Life Planning.
Vol. 26,
Issue. ,
p.
28.
Söngen, Hagen
Jaques, Ygor Morais
Spijker, Peter
Marutschke, Christoph
Klassen, Stefanie
Hermes, Ilka
Bechstein, Ralf
Zivanovic, Lidija
Tracey, John
Foster, Adam S
and
Kühnle, Angelika
2020.
Three-dimensional solvation structure of ethanol on carbonate minerals.
Beilstein Journal of Nanotechnology,
Vol. 11,
Issue. ,
p.
891.
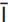 4} calcite surface: preliminary observations with atomic force microscopy
4} calcite surface: preliminary observations with atomic force microscopy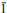 4} calcite surface cleaved in ethanol indicate a different surface behaviour than that of the {10
4} calcite surface cleaved in ethanol indicate a different surface behaviour than that of the {10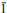 4} surface cleaved in air. The results are consistent with recent theoretical studies and suggest strong ordering of ethanol over the termination of the bulk calcite structure.
4} surface cleaved in air. The results are consistent with recent theoretical studies and suggest strong ordering of ethanol over the termination of the bulk calcite structure.

