Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Lipschutz, Michael E.
Wolf, Stephen F.
Hanchar, John M.
and
Culp, F. Bartow
2001.
Geochemical and Cosmochemical Materials.
Analytical Chemistry,
Vol. 73,
Issue. 12,
p.
2687.
Svendsen, Johan B
and
Hartley, Neil R
2002.
Synthetic heavy mineral stratigraphy: applications and limitations.
Marine and Petroleum Geology,
Vol. 19,
Issue. 4,
p.
389.
Catlos, E.J
Gilley, L.D
and
Harrison, T.Mark
2002.
Interpretation of monazite ages obtained via in situ analysis.
Chemical Geology,
Vol. 188,
Issue. 3-4,
p.
193.
Catlos, E. J.
Dubey, C. S.
Harrison, T. M.
and
Edwards, M. A.
2004.
Late Miocene movement within the Himalayan Main Central Thrust shear zone, Sikkim, north‐east India.
Journal of Metamorphic Geology,
Vol. 22,
Issue. 3,
p.
207.
2004.
Phoscorites and carbonatites from mantle to mine.
p.
341.
Catlos, E. J.
and
�emen, Ibrahim
2005.
Monazite ages and the evolution of the Menderes Massif, western Turkey.
International Journal of Earth Sciences,
Vol. 94,
Issue. 2,
p.
204.
González-Álvarez, Ignacio
Agnieszka Kusiak, Monika
and
Kerrich, Robert
2006.
A trace element and chemical Th–U total Pb dating study in the lower Belt-Purcell Supergroup, Western North America: Provenance and diagenetic implications.
Chemical Geology,
Vol. 230,
Issue. 1-2,
p.
140.
Catlos, Elizabeth J.
Shekhar Dubey, Chandra
Marston, Richard A.
and
Harrison, T. Mark
2007.
Convergent Margin Terranes and Associated Regions: A Tribute to W.G. Ernst.
Williams, Michael L.
Jercinovic, Michael J.
and
Hetherington, Callum J.
2007.
Microprobe Monazite Geochronology: Understanding Geologic Processes by Integrating Composition and Chronology.
Annual Review of Earth and Planetary Sciences,
Vol. 35,
Issue. 1,
p.
137.
Suzuki, Kazuhiro
and
Kato, Takenori
2008.
CHIME dating of monazite, xenotime, zircon and polycrase: Protocol, pitfalls and chemical criterion of possibly discordant age data.
Gondwana Research,
Vol. 14,
Issue. 4,
p.
569.
Popova, V. I.
and
Churin, E. I.
2010.
Zoning and sectoriality of monazite-(Ce) from granite pegmatites of the central and South Urals.
Geology of Ore Deposits,
Vol. 52,
Issue. 7,
p.
646.
Hetherington, Callum J.
Harlov, Daniel E.
and
Budzyń, Bartosz
2010.
Experimental metasomatism of monazite and xenotime: mineral stability, REE mobility and fluid composition.
Mineralogy and Petrology,
Vol. 99,
Issue. 3-4,
p.
165.
Stepanov, Aleksandr S.
Hermann, Joerg
Rubatto, Daniela
and
Rapp, Robert P.
2012.
Experimental study of monazite/melt partitioning with implications for the REE, Th and U geochemistry of crustal rocks.
Chemical Geology,
Vol. 300-301,
Issue. ,
p.
200.
Votyakov, S. L.
Khiller, V. V.
and
Shchapova, Yu. V.
2012.
Composition and chemical microprobe dating of U-Th-bearing minerals. Part I. Monazites from the Urals and Siberia.
Geology of Ore Deposits,
Vol. 54,
Issue. 8,
p.
625.
Chetty, D.
and
Gutzmer, J.
2012.
REE redistribution during hydrothermal alteration of ores of the Kalahari Manganese Deposit.
Ore Geology Reviews,
Vol. 47,
Issue. ,
p.
126.
Duraiswami, Raymond
and
Shaikh, Tahira
2014.
Fluid-rock interaction in the Kangankunde Carbonatite Complex, Malawi: SEM based evidence for late stage pervasive hydrothermal mineralisation.
Open Geosciences,
Vol. 6,
Issue. 4,
p.
476.
Laurent, Antonin T.
Seydoux-Guillaume, Anne-Magali
Duchene, Stéphanie
Bingen, Bernard
Bosse, Valérie
and
Datas, Lucien
2016.
Sulphate incorporation in monazite lattice and dating the cycle of sulphur in metamorphic belts.
Contributions to Mineralogy and Petrology,
Vol. 171,
Issue. 11,
Catlos, Elizabeth
and
Miller, Nathan
2017.
Speculations Linking Monazite Compositions to Origin: Llallagua Tin Ore Deposit (Bolivia).
Resources,
Vol. 6,
Issue. 3,
p.
36.
Broom-Fendley, Sam
Wall, Frances
Spiro, Baruch
and
Ullmann, Clemens V.
2017.
Deducing the source and composition of rare earth mineralising fluids in carbonatites: insights from isotopic (C, O, 87Sr/86Sr) data from Kangankunde, Malawi.
Contributions to Mineralogy and Petrology,
Vol. 172,
Issue. 11-12,
Chen, Wei
Honghui, Huang
Bai, Tian
and
Jiang, Shaoyong
2017.
Geochemistry of Monazite within Carbonatite Related REE Deposits.
Resources,
Vol. 6,
Issue. 4,
p.
51.
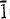 and {100} sectors; Ce shows no partitioning differences; and uptake of Nd is more easily facilitated on
and {100} sectors; Ce shows no partitioning differences; and uptake of Nd is more easily facilitated on 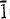 and {100} surfaces relative to {011}. There appears to be a distinct relationship between the size of the REE ion and the probability of uptake via the different growth surfaces. Interpretation of this uptake behaviour, based on theories involving ‘protosites’, involves an investigation of the possible kink site geometries at edge-steps during growth. Part-formed kink sites with small entrance sizes are calculated to occur with higher frequency on
and {100} surfaces relative to {011}. There appears to be a distinct relationship between the size of the REE ion and the probability of uptake via the different growth surfaces. Interpretation of this uptake behaviour, based on theories involving ‘protosites’, involves an investigation of the possible kink site geometries at edge-steps during growth. Part-formed kink sites with small entrance sizes are calculated to occur with higher frequency on 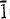 relative to {011}, and this correlates with an increase in the smaller-sized REE (Nd) uptake by
relative to {011}, and this correlates with an increase in the smaller-sized REE (Nd) uptake by 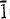 growth surfaces. The overall morphology and sector growth is suggested to be a function of uptake chemistry.
growth surfaces. The overall morphology and sector growth is suggested to be a function of uptake chemistry.

