Article contents
The crystal chemistry of the uranyl carbonate mineral grimselite, (K, Na)3Na[(UO2)(CO3)3](H2O), from Jáchymov, Czech Republic
Published online by Cambridge University Press: 05 July 2018
Abstract
Two crystals of the uranyl carbonate mineral grimselite, ideally K3Na[(UO2)(CO3)3](H2O), from Jáchymov in the Czech Republic were studied by single-crystal X-ray diffraction and electron-probe microanalysis. One crystal has considerably more Na than the ideal chemical composition due to substitution of Na into KO8 polyhedra; the composition of the other crystal is nearer to ideal, and similar to synthetic grimselite. The presence of Na atoms in KO8 polyhedra, which are located in channels in the crystal structure, reduces their volume, and as a result the unit-cell volume also decreases. Structure refinement shows that the formula for the sample with the anomalously high Na content is (K2.43Na0.57)Σ3.00Na[(UO2)(CO3)3](H2O). The unit-cell parameters, refined in space group P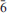 2c, are a = 9.2507(1), c = 8.1788(1) Å, V = 606.14(3) Å3 and Z = 2. The crystal structure was refined to R1 = 0.0082 and wR1 = 0.0185 with a GOF = 1.33, based on 626 observed diffraction peaks [Iobs>3σ(I)].
2c, are a = 9.2507(1), c = 8.1788(1) Å, V = 606.14(3) Å3 and Z = 2. The crystal structure was refined to R1 = 0.0082 and wR1 = 0.0185 with a GOF = 1.33, based on 626 observed diffraction peaks [Iobs>3σ(I)].
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2016
References
- 5
- Cited by




