Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Ivanyuk, Gregory
Kalashnikov, Andrey
Pakhomovsky, Yakov
Bazai, Ayya
Goryainov, Pavel
Mikhailova, Julia
Yakovenchuk, Victor
and
Konopleva, Nataly
2017.
Subsolidus Evolution of the Magnetite-Spinel-UlvöSpinel Solid Solutions in the Kovdor Phoscorite-Carbonatite Complex, NW Russia.
Minerals,
Vol. 7,
Issue. 11,
p.
215.
Ivanyuk, Gregory Yu.
Pakhomovsky, Yakov A.
Panikorovskii, Taras L.
Mikhailova, Julia A.
Kalashnikov, Andrei O.
Bazai, Ayya V.
Yakovenchuk, Victor N.
Konopleva, Nataly G.
and
Goryainov, Pavel M.
2018.
Three-D Mineralogical Mapping of the Kovdor Phoscorite-Carbonatite Complex, NW Russia: II. Sulfides.
Minerals,
Vol. 8,
Issue. 7,
p.
292.
Zhitova, Elena S.
Krivovichev, Sergey V.
Pekov, Igor
and
Greenwell, H. Christopher
2019.
Crystal chemistry of natural layered double hydroxides. 5. Single-crystal structure refinement of hydrotalcite, [Mg6Al2(OH)16](CO3)(H2O)4.
Mineralogical Magazine,
Vol. 83,
Issue. 02,
p.
269.
Gurzhiy, Vladislav V.
Tyumentseva, Olga S.
Izatulina, Alina R.
Krivovichev, Sergey V.
and
Tananaev, Ivan G.
2019.
Chemically Induced Polytypic Phase Transitions in the Mg[(UO2)(TO4)2(H2O)](H2O)4 (T = S, Se) System.
Inorganic Chemistry,
Vol. 58,
Issue. 21,
p.
14760.
Zhitova, Elena S.
Pekov, Igor V.
Chaikovskiy, Ilya I.
Chirkova, Elena P.
Yapaskurt, Vasiliy O.
Bychkova, Yana V.
Belakovskiy, Dmitry I.
Chukanov, Nikita V.
Zubkova, Natalia V.
Krivovichev, Sergey V.
and
Bocharov, Vladimir N.
2019.
Dritsite, Li2Al4(OH)12Cl2·3H2O, a New Gibbsite-Based Hydrotalcite Supergroup Mineral.
Minerals,
Vol. 9,
Issue. 8,
p.
492.
Zhitova, Elena S.
Krivovichev, Sergey V.
Pekov, Igor V.
and
Yapaskurt, Vasiliy O.
2019.
Crystal Chemistry of Chlormagaluminite, Mg4Al2(OH)12Cl2(H2O)2, a Natural Layered Double Hydroxide.
Minerals,
Vol. 9,
Issue. 4,
p.
221.
Zhitova, Elena S.
Greenwell, H. Chris
Krzhizhanovskaya, Mariya G.
Apperley, David C.
Pekov, Igor V.
and
Yakovenchuk, Victor N.
2020.
Thermal Evolution of Natural Layered Double Hydroxides: Insight from Quintinite, Hydrotalcite, Stichtite, and Iowaite as Reference Samples for CO3- and Cl-Members of the Hydrotalcite Supergroup.
Minerals,
Vol. 10,
Issue. 11,
p.
961.
Karpenko, Vladimir Yu.
Zhitova, Elena S.
Pautov, Leonid A.
Agakhanov, Atali A.
Siidra, Oleg I.
Krzhizhanovskaya, Maria G.
Rassulov, Victor A.
and
Bocharov, Vladimir N.
2020.
Akopovaite, Li2Al4(OH)12(CO3)(H2O)3, a new Li member of the hydrotalcite supergroup from Turkestan Range, Kyrgyzstan.
Mineralogical Magazine,
Vol. 84,
Issue. 2,
p.
301.
Zhitova, E.S.
Pekov, I.V.
Chukanov, N.V.
Yapaskurt, V.O.
and
Bocharov, V.N.
2020.
Minerals of the System Stichtite–Pyroaurite–Iowaite–Woodallite from Serpentinites of the Terekta Ridge (Gorny Altai, Russia).
Russian Geology and Geophysics,
Vol. 61,
Issue. 1,
p.
36.
He, Yan
Li, Weidong
Li, Jianan
Xu, Changsong
and
Lu, Xiaoke
2021.
Corrosion of Longquan celadons in the marine environment: study on the celadons from the Dalian Island shipwreck of the Yuan Dynasty.
Heritage Science,
Vol. 9,
Issue. 1,
Hawthorne, Frank C.
Mills, Stuart J.
Hatert, Frédéric
and
Rumsey, Mike S.
2021.
Ontology, archetypes and the definition of ‘mineral species’.
Mineralogical Magazine,
Vol. 85,
Issue. 2,
p.
125.
Yang, Hexiong
Gibbs, Ronald B.
Schwenk, Cody
Xie, Xiande
Gu, Xiangping
Downs, Robert T.
and
Evans, Stanley H.
2021.
Liudongshengite, Zn4Cr2(OH)12(CO3)·3H2O, a new mineral of the hydrotalcite supergroup, from the 79 mine, Gila County, Arizona, USA.
The Canadian Mineralogist,
Vol. 59,
Issue. 4,
p.
763.
Christy, Andrew G.
Pekov, Igor V.
and
Krivovichev, Sergey V.
2021.
The Distinctive Mineralogy of Carbonatites.
Elements,
Vol. 17,
Issue. 5,
p.
333.
Ke, Xinyuan
Baki, Vahiddin Alperen
Large, Alexander I.
Held, Georg
Walkley, Brant
and
Li, Jiaqi
2022.
Atomic-scale characterisation of sodium aluminosilicate hydrates (N-A-S-H) and Mg-substituted N(-M)-A-S-H using XANES.
Applied Geochemistry,
Vol. 147,
Issue. ,
p.
105515.
Gevers, Bianca R.
Roduner, Emil
and
Labuschagné, Frederick J. W. J.
2022.
Towards understanding photon absorption and emission in MgAl layered double hydroxide.
Materials Advances,
Vol. 3,
Issue. 2,
p.
962.
Kharitonskii, Petr
Bobrov, Nikita
Gareev, Kamil
Kosterov, Andrei
Nikitin, Andrey
Ralin, Andrey
Sergienko, Elena
Testov, Oleg
Ustinov, Alexander
and
Zolotov, Nikita
2022.
Magnetic granulometry, frequency-dependent susceptibility and magnetic states of particles of magnetite ore from the Kovdor deposit.
Journal of Magnetism and Magnetic Materials,
Vol. 553,
Issue. ,
p.
169279.
Gogoi, Rhituparna
and
Hazarika, Pranjit
2023.
Magmatic-hydrothermal evolution of the Sung Valley Carbonatite, northeastern India.
Journal of Earth System Science,
Vol. 132,
Issue. 2,
Zhitova, Elena S.
Sheveleva, Rezeda M.
Kasatkin, Anatoly V.
Zolotarev, Andrey A.
Bocharov, Vladimir N.
Kupchinenko, Anastasia N.
and
Belakovsky, Dmitry I.
2023.
Crystal Structure of Hydrotalcite Group Mineral—Desautelsite, Mg6MnIII2(OH)16(CO3)·4H2O, and Relationship between Cation Size and In-Plane Unit Cell Parameter.
Symmetry,
Vol. 15,
Issue. 5,
p.
1029.
Sudare, Tomohito
Ueda, Mizuki
Yamaguchi, Takuro
Tipplook, Mongkol
Tanaka, Hideki
Hayashi, Fumitaka
and
Teshima, Katsuya
2023.
Layer-Stacking Sequence Governs Ion-Storage in Layered Double Hydroxides.
The Journal of Physical Chemistry Letters,
Vol. 14,
Issue. 2,
p.
584.
Zhitova, Elena S.
Sheveleva, Rezeda M.
Zolotarev, Andrey A.
and
Krivovichev, Sergey V.
2023.
The Crystal Structure of Mg–Al–CO3 Layered Double Hydroxide.
Crystals,
Vol. 13,
Issue. 5,
p.
839.
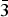 $\bar 3$c1, a = 5.2720(6), c = 15.113(3) Å and V = 363.76(8) Å3. The crystal structure consists of [Mg2Al(OH)6]+ brucite-type layers with an ordered distribution of Mg2+ and Al3+ cations according to the
$\bar 3$c1, a = 5.2720(6), c = 15.113(3) Å and V = 363.76(8) Å3. The crystal structure consists of [Mg2Al(OH)6]+ brucite-type layers with an ordered distribution of Mg2+ and Al3+ cations according to the 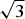 $\sqrt 3 $ ×
$\sqrt 3 $ × 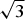 $\sqrt 3 $ superstructure with the layers stacked according to a hexagonal type. The complete layer stacking sequence can be described as …=Ab1C = Cb1A=…. The crystal structure of quintinite-3R was solved by direct methods and refined to R1 = 0.022 on the basis of 140 unique reflections. It is trigonal, R
$\sqrt 3 $ superstructure with the layers stacked according to a hexagonal type. The complete layer stacking sequence can be described as …=Ab1C = Cb1A=…. The crystal structure of quintinite-3R was solved by direct methods and refined to R1 = 0.022 on the basis of 140 unique reflections. It is trigonal, R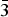 $\bar 3$m, a = 3.063(1), c = 22.674(9) Å and V = 184.2(1) Å3. The crystal structure is based upon double hydroxide layers [M2+,3+(OH)2] with disordered distribution of Mg, Al and Fe and with the layers stacked according to a rhombohedral type. The stacking sequence of layers can be expressed as …=АB = BC = CA=… The study of morphologically different quintinite generations grown on one another detected the following natural sequence of polytype formation: 2H → 2T → 1M that can be attributed to a decrease of temperature during crystallization. According to the information-based approach to structural complexity, this sequence corresponds to the increasing structural information per atom (IG): 1.522 → 1.706 → 2.440 bits, respectively. As the IG value contributes negatively to the configurational entropy of crystalline solids, the evolution of polytypic modifications during crystallization corresponds to the decreasing configurational entropy. This is in agreement with the general principle that decreasing temperature corresponds to the appearance of more complex structures.
$\bar 3$m, a = 3.063(1), c = 22.674(9) Å and V = 184.2(1) Å3. The crystal structure is based upon double hydroxide layers [M2+,3+(OH)2] with disordered distribution of Mg, Al and Fe and with the layers stacked according to a rhombohedral type. The stacking sequence of layers can be expressed as …=АB = BC = CA=… The study of morphologically different quintinite generations grown on one another detected the following natural sequence of polytype formation: 2H → 2T → 1M that can be attributed to a decrease of temperature during crystallization. According to the information-based approach to structural complexity, this sequence corresponds to the increasing structural information per atom (IG): 1.522 → 1.706 → 2.440 bits, respectively. As the IG value contributes negatively to the configurational entropy of crystalline solids, the evolution of polytypic modifications during crystallization corresponds to the decreasing configurational entropy. This is in agreement with the general principle that decreasing temperature corresponds to the appearance of more complex structures.



