Article contents
Crystal-chemical study of R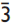 c natural oxides along the eskolaite – karelianite – hematite (Cr2O3–V2O3–Fe2O3) join
c natural oxides along the eskolaite – karelianite – hematite (Cr2O3–V2O3–Fe2O3) join
Published online by Cambridge University Press: 05 July 2018
Abstract
Six natural crystals from the Sludyanka crystalline complex belonging to the eskolaite (Cr2O3)–karelianite (V2O3)–hematite (Fe2O3) solid solution were studied by means of X-ray diffraction and electron microprobe. The Fe3+-poor samples show a general increase in a and c cell parameters with increasing mean cationic radius (MCR), consistent with that shown by the synthetic crystals along the eskolaite–karelianite join. The Fe3+-richer sample deviates significantly from the behaviour shown by the Fe3+-poor ones, similar to synthetic and natural hematites; with increasing MCR, the a and c cell parameters increase linearly along the eskolaite-karelianite join. However, for the samples rich in Fe3+, from karelianite to hematite, a shows a slightly steeper slope whereas the c parameter decreases strongly. The octahedral distortion increases slightly as a function of MCR along the eskolaite-karelianite join, whereas it increases markedly for Fe3+-rich samples. The evolution of the octahedral edges and of the octahedral distortions as a function of MCR are responsible for the behaviour of the unit-cell parameters along the eskolaite-karelianite-hematite join.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2008
References
- 4
- Cited by


