Introduction
Lichens are known to be extremely sensitive to changes in the chemical composition of the environment. In response to air pollution, there occurs a decrease in species diversity, alterations in morphology and physiology, and an accumulation of pollutants in thalli. Furthermore, many lichen species are highly affected by alterations to habitats indirectly caused by pollution, such as fragmentation and microclimate changes. Therefore, they are widely used as sensitive indicators and monitors of environmental quality (Nimis et al. Reference Nimis, Scheidegger and Wolseley2002).
In recent decades, technological advances have led to a global reduction in atmospheric emissions from industrial enterprises (Pacyna et al. Reference Pacyna, Pacyna, Fudala, Strzelecka-Jastrzab, Hlawiczka, Panasiuk and Friedrich2007; Kozlov et al. Reference Kozlov, Zvereva and Zverev2009), presenting a unique opportunity to study ecosystem resilience. The recovery of lichen communities after declines in pollution has been observed both in urban areas (Rose & Hawksworth Reference Rose and Hawksworth1981; Bates et al. Reference Bates, Bell and Farmer1990; Seaward & Letrouit-Galinou Reference Seaward and Letrouit-Galinou1991; Gilbert Reference Gilbert, Bates and Farmer1992) and in the surroundings of industrial enterprises (Beckett Reference Beckett and Gunn1995; Howe & Lendemer Reference Howe and Lendemer2011; Schram et al. Reference Schram, Wagner, McMullin and Anand2015; Mikhailova Reference Mikhailova2020). In all cases, the recovery has been very slow, taking decades. Some possible explanations for slow recolonization rates have been proposed. In urban areas, a decrease in emissions of one pollutant often coincides with an increase in another, continuously impacting lichens (e.g. a decrease in SO2 concentrations accompanied by a persistence or even an increase in NOx levels) (Bates et al. Reference Bates, Bell and Farmer1990; Loppi & Corsini Reference Loppi and Corsini2003). Another possible explanation is the higher sensitivity of lichen reproductive stages to pollution compared to mature thalli: diaspore production and establishment may be suppressed in formerly polluted areas (Margot Reference Margot, Ferry, Baddeley and Hawksworth1973; Gilbert Reference Gilbert, Bates and Farmer1992). Substratum contamination also poses a limiting factor for lichen re-establishment, with increased bark acidity impeding recolonization as observed on oaks in London, England (Bates et al. Reference Bates, Bell and Farmer1990). The importance of substratum contamination for diaspore development has been supported by laboratory experiments: the germination rate of Hypogymnia physodes (L.) Nyl. soredia was reduced when cultivated on metal-containing media (Marti Reference Marti1985; Hauck et al. Reference Hauck, Paul, Mulack, Fritz and Runge2002).
Based on the above studies, we conducted an experiment aimed at revealing possible factors limiting lichen recolonization in the formerly polluted area near the Middle Ural Copper Smelter (MUCS), one of the largest copper smelters in Russia, during the first decade after emission ceased. As a model species, we chose the foliose lichen Hypogymnia physodes, which dominates the epiphytic communities of the majority of tree species in the studied area. Previously, during the period of high emissions from MUCS (1995–1997), the response of this species to the pollution was studied in detail. Hypogymnia physodes abundance decreased along the pollution gradient, leading to the disappearance of the species at distances of 2–10 km from the smelter, depending on the wind rose (Mikhailova Reference Mikhailova2022). Under a strong pollution regime, a shift was observed in the population structure towards smaller and less sorediate thalli, reduced production of soredia, and decreases in soredial survival and developmental rates (Mikhailova & Vorobeichik Reference Mikhailova and Vorobeichik1999; Mikhailova & Scheidegger Reference Mikhailova and Scheidegger2001; Mikhailova Reference Mikhailova2002). This provided a solid basis for studying the recovery of H. physodes populations after emission cessation.
The aim of the present study was to assess the success of soredia development in a formerly polluted area near MUCS and to differentiate between the effects of bark properties and other environmental factors. Our hypothesis was that bark formerly exposed to long-term pollution might inhibit the early development of H. physodes. Consequently, we predict that this inhibition will be evidenced by reduced attachment efficiency, delayed growth, and lower survival rates of H. physodes on the polluted bark.
Materials and Methods
Study area and sample plots
The study was carried out in the surroundings of the Middle Ural Copper Smelter (Sverdlovsk Region of Russia, 56°51ʹN, 59°53ʹE) located in the southern taiga subzone on the western slope of the Middle Urals. The area is characterized by a warm-summer humid continental climate (Dfb, according to the Köppen-Geiger classification; Peel et al. Reference Peel, Finlayson and McMahon2007), with an annual air temperature of +2 °С, annual precipitation of 550 mm, and a snow-free period of c. 215 days. The area is covered by primary coniferous forests (Picea abies (L.) Karst., Abies sibirica Ledeb. and Pinus sylvestris L.) and secondary deciduous forests (Betula pendula Roth, B. pubescens Ehrh. and Populus tremula L.).
MUCS has been operational since 1940, emitting sulphur dioxide and dust particles with adsorbed metals (Cu, Fe, Zn, Pb, Cd, etc.) and metalloids (As). Total emission levels peaked at 225 000 t yr−1 in 1980, then decreased to 65 000–140 000 t yr−1 in 1990–1999, further reduced to 21 000–63 000 t/year in 2000–2009, and dropped to only 3000–5000 t/year after 2010. The long-term pollution caused degradation of forest ecosystems, described in a range of publications (Vorobeichik & Khantemirova Reference Vorobeichik and Khantemirova1994; Kaigorodova & Vorobeichik Reference Kaigorodova and Vorobeichik1996; Scheidegger & Mikhailova Reference Scheidegger, Mikhailova and Landolt2000). The most obvious signs of forest degradation were a thinned tree stand with a high proportion of dead trees and sparse herbaceous vegetation. In close proximity to the smelter (1 km), an industrial barren has formed, characterized by a degraded tree stand and eroded ground surface. Mapping of lichen communities on birch trunks between 1995–1997 revealed concentric zones of their gradual impoverishment when moving towards the MUCS. Within 2 km of the smelter, only 1–4 tolerant lichen species occurred sparsely (Scheidegger & Mikhailova Reference Scheidegger, Mikhailova and Landolt2000).
The experiment started in 2015, five years after almost complete cessation of emissions. Experimental sites were established in mixed fir-spruce forests (Abieto-Piceetum oxalidozum) in unpolluted (UP) and heavily polluted (HP) areas located 30 km and 1 km upwind of the smelter, respectively (three ecologically similar sample plots per area, spaced 200–500 m apart). In the unpolluted plots, Aegopodium podagraria L., Ajuga reptans L., Calamagrostis arundinacea (L.) Roth, Cerastium pauciflorum Stev. ex Ser., Circaea alpina L., Dryopteris spp., Maianthemum bifolium (L.) F. W. Schmidt, Oxalis acetosella L., dominated in ground-level vegetation (Vorobeichik et al. Reference Vorobeichik, Trubina, Khantemirova and Bergman2014). Lichen communities on fir trunks were dominated by Chaenotheca ferruginea (Turner ex Sm.) Mig. and Hypogymnia physodes. Other common members of the communities were Сladonia coniocraea (Flörke) Spreng., Lepraria spp. and Xylopsora caradocensis (Leight. ex Nyl.) Bendiksby & Timdal. Species with a high sensitivity to pollution were also present including Bryoria nadvornikiana (Gyeln.) Brodo & D. Hawksw., Evernia mesomorpha Nyl., and Usnea subfloridana Stirt. (Mikhailova Reference Mikhailova2020).
In the heavily polluted area, sample plots were established in the forest remnants closest to the smelter. At the beginning of the experiment, there was no evidence of forest recovery, despite the cessation of emissions. Ground-level vegetation consisted of a few species, such as Agrostis capillaris L., Brachypodium pinnatum (L.) Beauv., Deschampsia caespitosa (L.) Beauv., Equisetum sylvaticum L., Lathyrus vernus (L.) Bernh., Sanguisorba officinalis L., Vaccinium myrtillus L. and V. vitis-idaea L. (Vorobeichik et al. Reference Vorobeichik, Trubina, Khantemirova and Bergman2014). The ground was covered by a thick layer of nearly undecomposed forest litter (Vorobeichik & Kaigorodova Reference Vorobeichik and Kaigorodova2017). However, several lichen species began to re-invade the area. In 2014, five lichen species were recorded on fir trunks 1 km from the smelter, and over the next five years the number of species increased to eight. Nevertheless, the composition of lichen communities radically differs from that in the unpolluted area, with a dominance of X. caradocensis and an increased abundance of toxitolerant species such as Placynthiella icmalea (Ach.) Coppins & P. James and Scoliciosporum chlorococcum (Graewe ex Stenh.) Vezda. Hypogymnia physodes was listed as one of the active colonizers of this area, although its abundance was still three orders of magnitude lower than in the UP area (Mikhailova Reference Mikhailova2020).
Data collection
Sowing of soredia was performed according to the method described by Mikhailova & Scheidegger (Reference Mikhailova and Scheidegger2001). Healthy dry thalli of H. physodes were collected from fir trunks in the UP area, placed in plastic bags and kept in the laboratory at room temperature for 2 days before sowing. Bark squares (c. 1 cm2) were cut from the bases (up to 30 cm) of fir trunks in the UP and HP localities. In the laboratory, the bark pieces were carefully cleaned of lichens under a dissecting microscope and glued onto 15 cm long wooden bars, with 10 pieces per bar. The bark surface was slightly moistened with a paintbrush immediately before sowing. Soredia of H. physodes were collected from the plastic bags using a wet paintbrush and transferred onto the bark squares ensuring uniform surface coverage. Five fir trees were chosen per plot, and two bars (one bearing bark from the HP area and the other from the UP area) were attached vertically to the bases of the trunks using plastic ribbon. A total of 600 bark pieces were established (2 areas × 3 plots × 5 trees × 2 bars × 10 bark pieces), resulting in four treatment types: UP area × bark from the UP area (UPA × UPB); UP area × bark from the HP area (UPA × HPB); HP area × bark from the UP area (HPA × UPB); and HP area × bark from the HP area (HPA × HPB).
After sowing in May 2015, soredia survival was evaluated in June and September 2015. In 2016 and 2017, survival was assessed twice annually, in June and October, and the final assessment was performed in June 2018. For these evaluations, bars were transported to the laboratory, where the number of bark pieces with developing soredia was counted using a dissecting microscope. Mikhailova & Scheidegger (Reference Mikhailova and Scheidegger2001) described the following stages of H. physodes soredia development: soredial, granular, thallus primordium, lobulate, and branched lobes. In the present study, we distinguished between the pre-thallus (soredial, granular and thallus primordium) and thallus (simple and branched lobes) stages. In the first year of the experiment (2015–2016), only pre-thallus stages were observed on the experimental bark squares. By June 2017, the first juvenile lobes developed, marking the experiment's transition to its second stage. Thereafter, we counted only bark squares with lobes, considering lobe formation as the final indicator of successful development. When simple lobes started to branch, the number of bark squares with branched lobes was additionally counted. As an estimate of the survival rate, we used the proportion of bark pieces bearing soredia or lobes.
Data analysis
To assess the association between the probability of soredia survival or lobe development and various factors including pollution area, bark type and observation period, we employed a mixed-effects logistic regression model. The model incorporated the Bernoulli conditional distribution for the response variable and a logit link function (Bolker Reference Bolker, Fox, Negrete-Yankelevich and Sosa2015). The selection of the Bernoulli distribution was based on its suitability for binary outcome variables, with the use of absolute numbers over percentages allowing for a more accurate assessment of uncertainty in survival rate estimates. The model was structured to examine the interactions between pollution area, bark type and observation period as fixed effects. The unique identifiers for the sampling site and the tree holding bars with bark pieces were included as group-level (random) effects. The analysis was conducted using R v. 4.1.0 (R Core Team 2023) with brms v. 2.15.0 (Bürkner Reference Bürkner2017) interfacing with Stan v. 2.21.2 (Carpenter et al. Reference Carpenter, Gelman, Hoffman, Lee, Goodrich, Betancourt, Brubaker, Guo, Li and Riddel2017). We ran eight Markov chain Monte Carlo (MCMC) simulations, each with 12 500 sampling iterations and 2500 warm-up iterations. The convergence of MCMC chains was assessed both visually and quantitatively, using the improved potential scale reduction factor (R-hat diagnostic test) as proposed by Vehtari et al. (Reference Vehtari, Gelman, Simpson, Carpenter and Bürkner2021), along with the effective sample size (ESS) metric. Additional model fitting parameters included an adaptation delta set at 0.98 and a maximum tree depth of 10, which enhanced sampling efficiency and model convergence. Posterior estimates of marginal means were computed using emmeans v. 1.6.0 (Lenth Reference Lenth2023). For pairwise comparisons among different treatment levels, we conducted Tukey's honest significant difference tests and estimated log odds ratios. These comparisons, conducted on the estimated marginal means, allowed for the assessment of treatment effects across the various levels of predictors. For visualizing the results, we used ggplot2 v. 3.3.4 (Wickham Reference Wickham2016).
Results
Pre-thallus stages
After one month of cultivation, in June 2015, all bark pieces were still covered by soredia. From June 2015 to September 2016, a gradual loss of soredia occurred, with varying loss rates among treatment types (Fig. 1A).
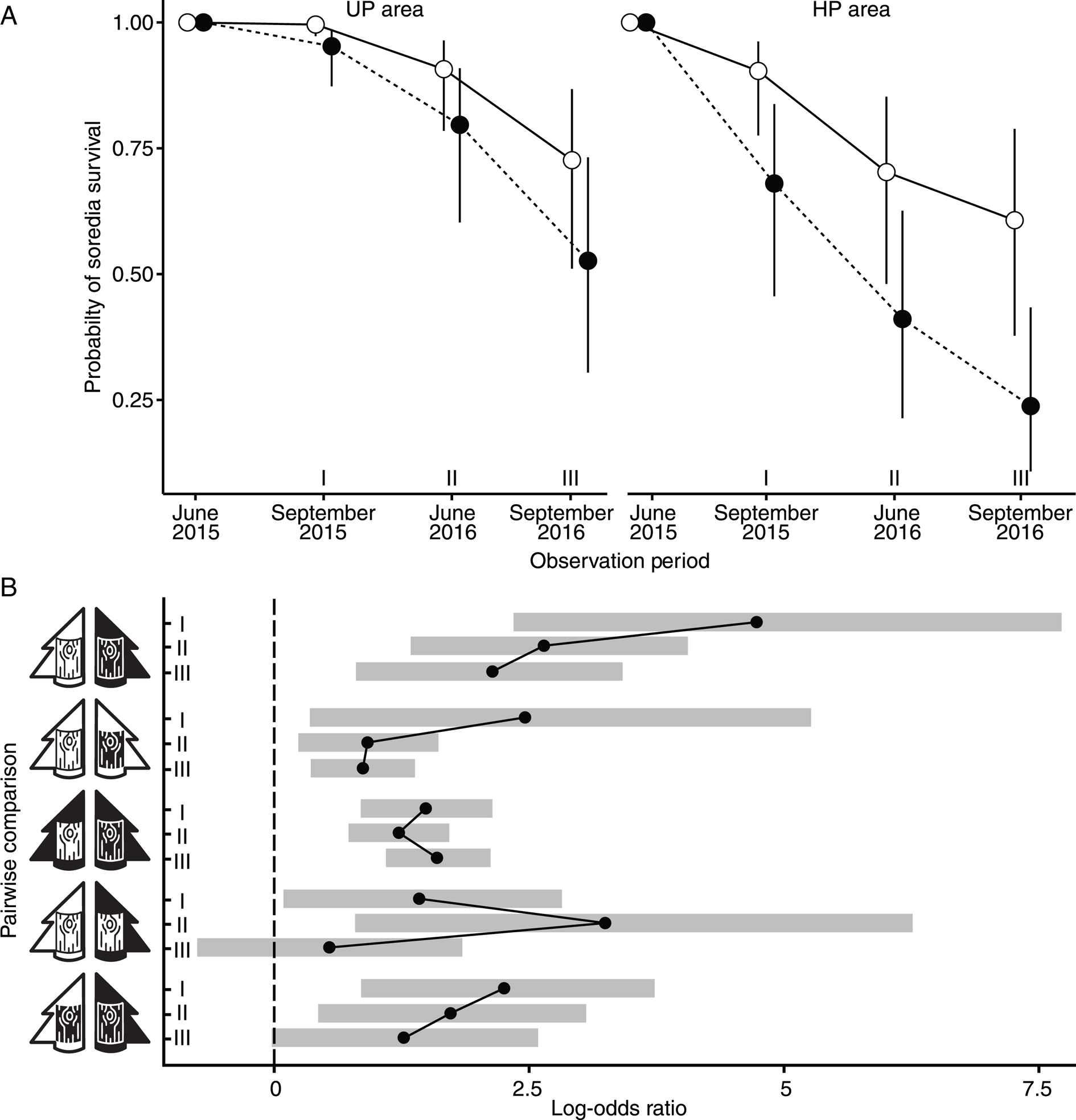
Figure 1. A, the probability of Hypogymnia physodes soredia survival in unpolluted (UP) and heavily polluted (HP) areas on both unpolluted (open marks) and polluted (filled marks) bark. The dots represent median values, while the whiskers denote the 95% credible intervals. B, pairwise comparisons of survival rates across different observation periods (labelled as I, II and III, corresponding to the respective observation dates, in panel A). The dots indicate the estimated log odds ratios, where a value of zero implies no difference between the compared groups, and positive values suggest increased survival rates in the first group of the comparison. The shaded areas around each dot depict the 95% credible intervals, and lines are used to connect comparisons within the same pair of treatment types, facilitating comparison of trends across the observation periods. The white tree represents the unpolluted area (UPA), while the black tree symbolizes the heavily polluted area (HPA); similarly, white bark indicates bark from the unpolluted area (UPB), and black bark denotes bark from the heavily polluted area (HPB). The study was carried out in the surroundings of the Middle Ural Copper Smelter, Sverdlovsk Region of Russia.
Cultivation on bark of the same habitat
Under the UPA × UPB treatment, the proportion of soredia-bearing bark pieces remained almost unchanged during 4 months of cultivation (by September 2015) and decreased to 0.91 by June 2016 (median values reported here and below). Under the HPA × HPB treatment, the proportion of soredia-bearing bark pieces had already decreased to 0.68 after the cultivation period of 4 months (Fig. 1A). Pairwise comparison between HPA × HPB and UPA × UPB treatment types confirmed a significant decrease in the probability of soredia survival in the highly polluted area throughout the experiment, with the difference in magnitude increasing over time (Fig. 1B). By September 2016, the difference between soredia survival in the UP and HP areas tripled (median survival values were 0.24 and 0.73, respectively). The highest odds ratios were observed for the comparison of HPA × HPB and UPA × UPB treatment types (Fig. 1B).
Differences in soredia survival between UPA × UPB and HPA × HPB treatment types were attributed to the combined effect of bark properties and other environmental factors. To differentiate the effect of bark properties, we examined treatment types involving ‘bark of different origin in the same pollution area’ and ‘bark of the same origin in different pollution areas’.
Cultivation on bark of different origin in the same pollution area
In the UP area, the probability of soredia survival on the native bark was significantly higher than on the bark from the HP area throughout the first stage of the experiment (Fig. 1A & B). The difference between median survival values increased over time, reaching 0.2 by September 2016. The same pattern was found in the polluted localities (HPA × HPB vs HPA × UPB), where differences in soredia survival on the bark of different origin were even more pronounced than in the UP area (Fig. 1A). In September 2015, median survival values on the UP bark exceeded that on the HP bark by 0.22, and by September 2016 the difference between barks of different origin reached 0.37, underscoring the importance of bark properties for diaspore survival when other site parameters were identical.
Cultivation on bark of the same origin in different pollution areas
Soredia survival on UP bark was significantly higher in the UP area compared to the HP localities in September 2015 and July 2016, although the magnitude of the difference was not comparatively high (0.09 and 0.20, respectively). Subsequently, the estimates levelled off (Fig. 1A).
Soredia survival on HP bark in the UP area was consistently higher than in the HP area throughout the first stage of the experiment (in October 2016, survival in the UP area was around twice as high (Fig. 1A)), although the odds ratio decreased over time (Fig. 1B).
Formation of stratified lobes
After two years of the experiment (June 2017), simple lobes were registered on the bark pieces under all treatment types (Fig. 2A). The proportion of bark pieces bearing lobes increased until the end of the experiment under all treatment types, except for HPA × UPB. These results indicate that the pre-thallus developmental stages remained viable for more than two years and were capable of further development. These observations are similar to our previous study, where granular stages persisted up to 29 months during the experiment (Mikhailova & Scheidegger Reference Mikhailova and Scheidegger2001).
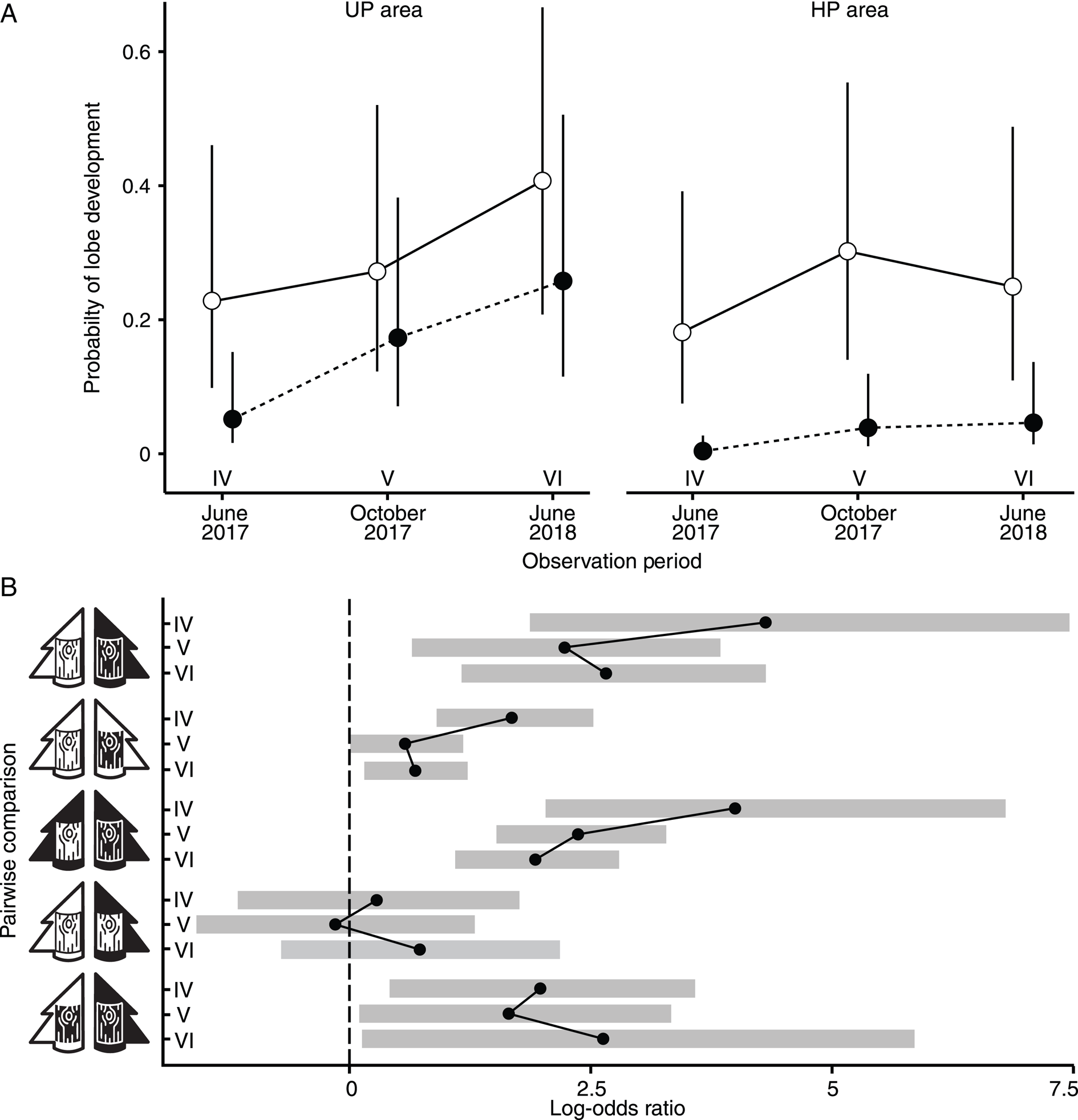
Figure 2. A, the probability of Hypogymnia physodes lobe development in UP and HP areas on unpolluted (open marks) and polluted (filled marks) bark. B, pairwise comparisons of lobe development rates across different observation periods (IV–VI, as denoted in panel A). The pictograms for bark and tree are consistent with those introduced in Fig. 1.
Cultivation on bark from the same habitat
A pairwise comparison between UPA × UPB and HPA × HPB treatment types revealed a higher probability of lobe formation in the UP area from the beginning of lobe formation until the end of the experiment (Fig. 2B). Under the HPA × HPB treatment type, the lowest probability of lobe formation was observed (< 0.1 by the end of the experiment).
Cultivation on bark of different origin in the same pollution area
In the UP area, the probability of lobe formation on UP bark was consistently higher than on HP bark throughout the experiment (Fig. 2A). This trend was also observed in the HP area: UP bark was more conducive to lobe development, as indicated by the highest odds ratio (Fig. 2B). A slight decrease in the probability of lobe formation on the UP bark in the HP area was noted between October 2017 and June 2018, but this was not statistically significant.
Cultivation on bark of the same origin in different pollution areas
The development of lobes on UP bark was not significantly affected by the pollution regime during any observation period (Fig. 2). By contrast, the pollution regime significantly influenced the probability of lobe formation on HP bark (with a higher probability observed on the UP plots).
Branching of lobes
The first branching lobes were registered under all treatment types in October 2017, after 30 months of cultivation (Fig. 3A). Pairwise comparison confirmed a higher probability of branching under the UPA × UPB treatment compared to HPA × HPB by the end of the experiment (June 2018), although even in October 2017 the confidence interval of the odds ratio was already skewed towards positive values (Fig. 3B). In the UP area, the effect of bark origin was significant only in June 2018. In the HP area, however, a significantly higher probability of branching on the UP bark was registered as early as October 2017 and persisted until the end of the experiment.
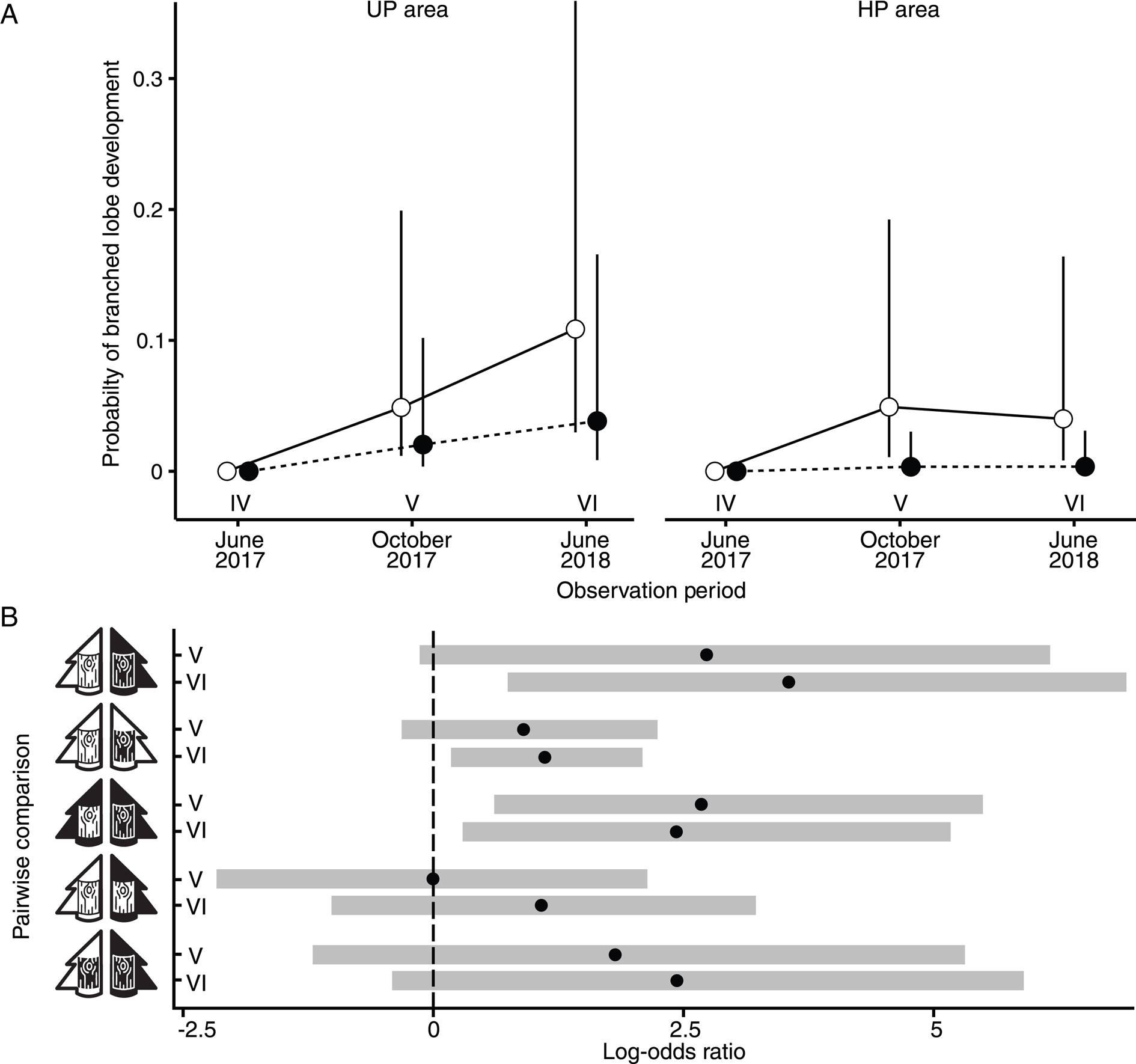
Figure 3. A, the probability of branched lobe development in Hypogymnia physodes in UP and HP areas on unpolluted (open marks) and polluted (filled marks) bark. B, pairwise comparisons of branched lobe development rates across different observation periods (IV–VI, as denoted in panel A). The pictograms for bark and tree are consistent with those introduced in Fig. 1.
Pollution area did not significantly affect the probability of lobe branching on either the polluted or unpolluted bark (UPA × HPB vs HPA × HPB, UPA × UPB vs HPA × UPB), although the log odds ratio was positive by the end of the experiment, and the confidence interval shifted towards positive values (Fig. 3B).
Along with the development of stratified lobes from pre-thallus stages, we observed the loss of formed lobes, that is, instances where lobes initially appeared on a bark square but were absent at the subsequent observation. From October 2017 to June 2018, the proportion of these bark squares in the HP area was higher than in the UP area (Table 1). Comparing the losses of formed lobes on unpolluted bark between the areas using Fisher's exact test revealed significant differences (P < 0.001): 28.26% in the HP area versus 4.88% in the UP area. Loss of formed lobes on the HP bark in the HP area also exceeded that in the UP area, but the extremely low number of bark samples bearing lobes under the HPA × HPB treatment type makes significance testing challenging.
Table 1. Loss of juvenile lobes of Hypogymnia physodes from October 2017 to June 2018. The study was carried out in the surroundings of the Middle Ural Copper Smelter, Sverdlovsk Region of Russia. UPA = unpolluted area; UPB = unpolluted bark; HPA = heavily polluted area; heavily polluted bark = HPB
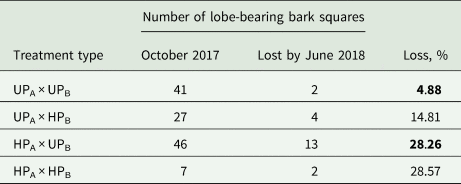
Significantly different comparison pairs are highlighted in bold (exact Fisher's test, P < 0.001)
Table 2 presents the proportion of bark pieces with developed lobes by the end of the experiment. The most contrasting treatment types (UPA × UPB and HPA × HPB) exhibited the most divergent results, with other treatment types falling in between.
Table 2. The success of the early development of Hypogymnia physodes under different treatment types by the end of the experiment. The study was carried out in the surroundings of the Middle Ural Copper Smelter, Sverdlovsk Region of Russia. UPA = unpolluted area; UPB = unpolluted bark; HPA = heavily polluted area; heavily polluted bark = HPB
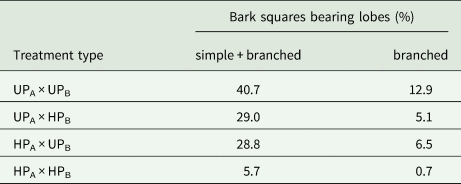
Thus, despite the almost complete cessation of emissions, the establishment and development of soredia were less successful in the formerly polluted environment compared to unpolluted areas. Our attempt to distinguish the effect of substratum properties from other habitat factors highlighted the importance of bark origin for the success of soredia development at all stages of early development, from attachment to the substratum to the formation of branched lobes.
Discussion
Cultivating soredia in the field presents a valuable tool for enhancing our understanding of the establishment and early development of lichens. It allows for both a detailed description of developmental stages and a quantitative assessment of survivability and developmental rates in different environments. The development of Hypogymnia physodes from soredia has already been studied experimentally in Europe (Schuster Reference Schuster1985; Ott Reference Ott1987a, Reference Ottb) and this allows us to compare development rates between different climatic zones. In the oceanic climate, lobe formation occurred within 11–16 months (Ott Reference Ott1987a, Reference Ottb), while in the subcontinental climate of Germany it took 17–25 months (Schuster Reference Schuster1985). In the continental climate of the Middle Urals, the developmental rates of H. physodes diaspores are notably slow. In non-polluted birch stands, simple lobes were registered only after 16 months of cultivation and, even after 29 months, most remained unbranched and under 0.5 mm in length (Mikhailova & Scheidegger Reference Mikhailova and Scheidegger2001). The present study revealed even slower development in fir and spruce forests, with the first simple lobes appearing only after two years of cultivation. We suggest the short snowless period (of 5–6 months) in the Middle Urals to be the main reason for these slow developmental rates. The lower light intensity in fir and spruce forests, coupled with the unique properties of fir bark, seem to further impede soredia development.
This study showed that soredia development in the HP area was more successful following the cessation of emissions compared to the high emission period. Juvenile lobes survived there until the end of the experiment (i.e. for 38 months of cultivation), with some showing potential for further branching and development. In a study undertaken during the high emission period, between 1995–1997, simple lobes were registered after 16 months of cultivation in the lichen desert area. However, none survived the following nine months, even though they were cultivated on unpolluted bark (Mikhailova & Scheidegger Reference Mikhailova and Scheidegger2001).
Nevertheless, even a decade after emissions ceased, our results showed that there were negative impacts on the early development of H. physodes in the formerly polluted area. This was particularly evident when soredia were cultivated on polluted bark, indicating a combined effect of bark properties and other environmental factors.
When discussing bark properties, we first consider bark chemistry, which is well known to be clearly affected by pollution (Chrabąszcz & Mróz Reference Chrabąszcz and Mróz2017). In the former lichen desert near the MUCS, total Cu concentration in fir bark, although lower since the high emission period, was still 7.5 times higher than in the unpolluted area. Moreover, bark pH remained 0.3 units lower than in unpolluted localities (Mikhailova Reference Mikhailova2020). The current elemental composition of the bark probably originates from multiple sources. Some elements have persisted since the high emission period, and the deposition of dust from surface soil and forest litter onto the bark is highly probable in the post-emission period, as evidenced by studies such as Loppi et al. (Reference Loppi, Nelli, Ancora and Bargagli1997). By the time of the current study, metal content in forest litter and upper soil in the former lichen desert had remained stable or even increased since the high emission period, except for Cu, which decreased significantly but was still well above the background levels (100 times greater for forest litter and 20 times for upper soil (Vorobeichik & Kaigorodova Reference Vorobeichik and Kaigorodova2017)). These observations concur with the general view on long-term persistence of metals in soil, ranging from 100 to 700 years, after emissions have ceased (Tyler Reference Tyler1978). Despite numerous studies on bark and lichen chemistry, our understanding of element mobility in bark remains limited. Therefore, it is challenging to determine the proportions of elements in a bound state versus those available to lichens and how these proportions change over time. Nevertheless, the cessation of air pollution does not completely eliminate the toxic impact on epiphytes, which persists, at least partially, through bark chemistry. Our data indicate a clear negative effect of bark from the polluted habitats on the success of early development of H. physodes. This impact was evident in all stages of soredia development, leading to a lower probability of juvenile lobe formation on polluted bark, even when soredia were cultivated in non-polluted habitats. In the conditions of the former lichen desert, bark properties were even more critical and have been exacerbating the impact of other habitat factors.
Among other environmental factors influencing soredia development, the altered microclimate of habitats should be considered foremost. The recovery of vascular plants in close proximity to the smelter has been markedly slow due to heavy metal excess in the upper soil (Vorobeichik et al. Reference Vorobeichik, Trubina, Khantemirova and Bergman2014). Pollution-induced thinning of tree stands and the poor ground vegetation has led to a slight ‘aridization’ of the microclimate: at a height of 2 m above ground, the daily average temperature was 0.7–1.0 °C higher than in the unpolluted localities (Belskii & Belskaya Reference Belskii and Belskaya2021). In the herbaceous layer, this difference was even more pronounced, with the maximum daily temperature being 3 °С higher in polluted localities than in the unpolluted area (Zolotarev & Nesterkov Reference Zolotarev and Nesterkov2015). Such temperature increases lead to lower humidity levels, potentially affecting the attachment and development of soredia. Under drier conditions, soredia may fail to germinate promptly, increasing the risk of being blown from the substratum (Schuster Reference Schuster1985; Schuster et al. Reference Schuster, Ott and Jahns1985).
The experimental design of the present study allowed us to differentiate between groups of factors designated as ‘bark properties’ and ‘other environmental factors’ regarding their impact on soredia development. However, exact identification of factors within these groups is not possible and needs further clarification. It is not only the hydrothermic regime of habitats that is responsible for the lower survival of soredia and, in particular, for the higher mortality of formed juvenile lobes in the formerly polluted area. Although the emissions are extremely low, they can still affect lichens in close proximity to the smelter. Bark characteristics, other than acidity and metal content (such as density, water capacity, surface characteristics, etc.), may also play a role, although we did not find any data on changes in these parameters in the polluted environment. Long-term studies of bark chemistry following the cessation of emissions are necessary for a better understanding of recovery patterns in epiphytic communities.
Conclusion
Our study supports the initial hypothesis: bark characteristics are of great importance for soredia development in the formerly polluted areas. The environment near the smelter has improved since the cessation of emissions, as evidenced by the more successful soredia development compared to the period of high emissions. Recolonization may occur more rapidly on younger trees that have not been exposed and thus have bark free of pollutants.
However, environmental conditions remain suboptimal for the development of Hypogymnia physodes. For the complete restoration of lichen communities, the recovery of a habitat's microclimate is essential.
Acknowledgements
We express our gratitude to Christoph Scheidegger, in collaboration with whom the method of soredia cultivation was developed and tested, and to Eugene Vorobeichik for stimulating discussions related to the current research. We are also grateful to anonymous reviewers for their valuable comments. The manuscript was prepared under the State Assignment of the Institute of Plant and Animal Ecology, Ural Branch, Russian Academy of Sciences (contract no. 122021000076-9).
Author ORCIDs
Irina Mikhailova, 0000-0002-0056-2295; Vladimir Mikryukov, 0000-0003-2786-2690.
Competing Interests
The authors declare none.








