Introduction
The genus Astrothelium Eschw. includes pyrenocarpous lichen-forming fungi within Trypetheliaceae (Harris Reference Harris1984, Reference Harris1995). Originally restricted to species with lateral, fused ostioles and transversely septate ascospores, in its revised delineation it comprises the majority of species in the Trypetheliaceae (Aptroot & Lücking Reference Aptroot and Lücking2016), with variable ascoma arrangement and ascospore septation. In both its traditional and its current circumscription, the genus has a pantropical distribution (Harris Reference Harris1984; Makhija & Patwardhan Reference Makhija and Patwardhan1989; Awasthi Reference Awasthi1991; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Kirk et al. Reference Kirk, Cannon, Minter and Stalpers2008; Aptroot Reference Aptroot2009; Hyde et al. Reference Hyde, Liu, Binder, Aryawansha, Boehm, Boonmee, Braun, Chomnunti, Crous and Dai2013).
Studies on species diversity in taxa with astrothelioid ascomata and ascospores in Astrothelium (i.e. Astrothelium in its previous sense) have focused mostly on neotropical regions (Malme Reference Malme1924; Harris Reference Harris1984; Reference Harris1995; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Lücking et al. Reference Lücking, Seavey, Common, Beeching, Breuss, Buck, Crane, Hodges, Hodkinson and Lay2011; Lima et al. Reference Lima, Maia, Aptroot and Cáceres2013; Córdova-Chávez et al. Reference Córdova-Chávez, Aptroot, Castillo-Camposa, Cáceres and Pérez-Pérez2014). In contrast, the diversity of this group in the Indomalayan area is far less known, with the exception of the Indian subcontinent (Makhija & Patwardhan Reference Makhija and Patwardhan1988, Reference Makhija and Patwardhan1989; Awasthi Reference Awasthi2000; Singh & Sinha Reference Singh and Sinha2010; Weerakoon & Aptroot Reference Weerakoon and Aptroot2014). Some of the species within this group found in the Indian subcontinent are believed to be endemic to the area (Makhija & Patwardhan Reference Makhija and Patwardhan1989; Weerakoon & Aptroot Reference Weerakoon and Aptroot2014). The diversity of Astrothelium species with astrothelioid ascomata and ascospores in Thailand is poorly known, with only four species recorded: A. cinnamomeum (Eschw.) Müll. Arg., A. eustomum (Mont.) Müll. Arg., A. galbineum Kremp., and A. variolosum (Ach.) Müll. Arg. (Vongshewarat Reference Vongshewarat2000; Aptroot et al. Reference Aptroot, Saipunkaew, Sipman, Sparrius and Wolseley2007). These taxa are either widely distributed in South-East Asia or are pantropical (Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008).
The aim of this study was to investigate the diversity of astrothelioid species of Astrothelium in Thailand, including taxonomy, chemistry and molecular data. We found the diversity to be higher than previously understood, with five species described here new to science and one new record for Thailand.
Material and Methods
Specimen collection, identification and mycobiont isolation
Specimens were collected from tropical rainforest and submontane evergreen forests in several locations in Thailand (Table 1). Cross-sections were mounted in tap water and investigated using an Olympus SZ11 stereomicroscope and Olympus BX41 compound microscope with differential interference contrast (DIC) (Olympus U-DICT), connected to a Canon EOS650 digital camera. Secondary metabolites were examined by colour spot test (10% KOH, saturated solution NaClO and p-phenylenediamine dissolved in ethanol), reaction under long-wave UV light (360 nm), and thin-layer chromatography (TLC) using solvent A (Culberson Reference Culberson1972; Lumbsch Reference Lumbsch2002).
Table 1 Species and specimens used in this study, with vouchers, location, herbarium (RAMK) and GenBank Accession numbers. Newly generated sequences are in bold. *=sequences from lichen thalli
The lichen mycobionts were isolated from fresh material by the ascospore discharge technique (Sangvichien et al. Reference Sangvichien, Hawksworth and Whalley2011). Ascospore germination and cultivation of the mycobiont was carried out on Malt-Yeast Extract medium incubated at room temperature (32–35 °C). Fifteen mycobionts developed colonies after 4–6 weeks that were used in this study. Lichen specimens and mycobiont cultures were deposited at the Lichen Herbarium of Ramkhamhaeng University (RAMK), Bangkok.
DNA extraction and PCR amplification
Fragments of mycobiont cultures were used for genomic DNA extraction using the CTAB method as modified by Cubero & Crespo (Reference Cubero and Crespo2002). DNA amplification was performed for four nucleotide markers: 1) internal transcribed spacer (ITS), 2) nuclear large subunit ribosomal DNA (nuLSU), 3) mitochondrial small subunit ribosomal DNA (mtSSU), and 4) the largest subunit of RNA polymerase II (RPB1) using the following primers: 1) ITS1F (Gardes & Bruns Reference Gardes and Bruns1993) with ITS4 (White et al. Reference White, Bruns, Lee and Taylor1990), 2) LR0R with LR3 (Vilgalys & Hester Reference Vilgalys and Hester1990), 3) mrSSU1 (Zoller et al. Reference Zoller, Scheidegger and Sperisen1999) with MSU7 (Zhou & Stanosz Reference Zhou and Stanosz2001), and 4) RPB1-Af with RPB1-Cr (Matheny et al. Reference Matheny, Liu, Ammirati and Hall2002).
The 50 µl PCR reaction consisted of 5 µl 10× Pfu Buffer with MgSO4, 2 mM of dNTP mix, 20 µM of each primer, 1·25 U of Pfu DNA Polymerase (Thermo Fisher Scientific Inc.) and 5 µl of 1/10 dilution of DNA solution. PCR conditions were as follows: initial denaturation for 1 min at 94°C and 38 cycles of 94°C for 1 min, 51°C for 1 min (ITS1F/ITS4), 52°C for 45s (LR0R/LR3), 53°C for 45s (mrSSU1/MSU7) and 52°C for 1·30 min (RPB1-Af/RPB1-Cr), followed by an extension at 72°C for 1 min, and a final extension at 72°C for 5 min. The samples were detected under UV light using agarose gel electrophoresis containing DNA Stain G (SERVA). The Gel/PCR DNA Fragments Extraction Kit (Genaid, Taiwan) was used to clean up the PCR products, according to the manufacturer’s instructions, and DNA was sequenced at 1st BASE Laboratories (Malaysia).
Sequence alignments and phylogenetic analyses
DNA sequences were aligned using MUSCLE (Edgar Reference Edgar2004) and manually adjusted using MEGA v.6 software (Tamura et al. Reference Tamura, Stecher, Peterson, Filipski and Kumar2013). Two samples of Bathelium madreporiforme were selected as outgroup. Single locus analyses (data not shown) did not show conflict, hence a concatenated dataset from our loci was used (Table 1). The nucleotide substitution model was determined using jModelTest v.2.1.4 (Darriba et al. Reference Darriba, Taboada, Doallo and Posada2012) with the Akaike Information Criterion (AIC). The GTR+I+G model was chosen for phylogenetic tree reconstruction through maximum likelihood (ML) and Bayesian inference (BI). Maximum likelihood analyses were carried out using RAxML-HPC2 v.8.2.4 (Stamatakis et al. Reference Stamatakis, Hoover and Rougemont2008) on the Cipres Web Portal (https://www.phylo.org) and bootstrap values were calculated with 1000 pseudoreplicates. Bayesian analysis was performed using MrBayes v.3.2.1 (Ronquist & Huelsenbeck Reference Ronquist and Huelsenbeck2003) for 10 million generations with two independent runs of four chains. Tree samples were saved every 100th trees and the mean standard deviation of split frequencies <0·01. Additionally, maximum parsimony (MP) trees were estimated using PAUP* v.4.0b10 (Swofford Reference Swofford1999) with a heuristic search algorithm and bootstrap values were calculated using 1000 replicates. Phylogenetic trees were visualized using FigTree v.1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
Results and Discussion
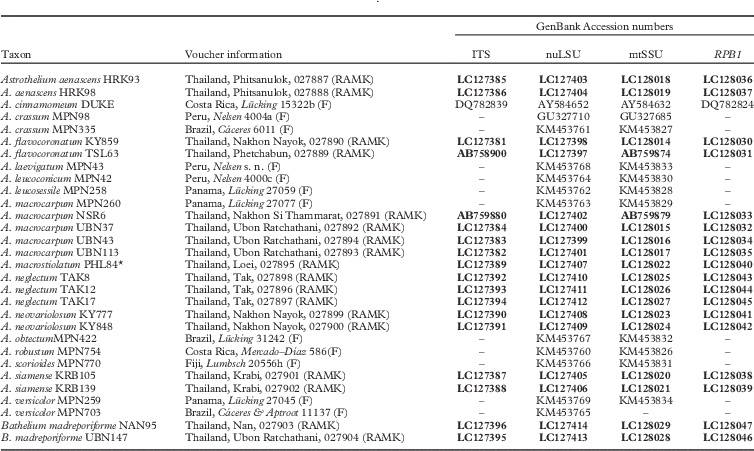
Seventy-two new DNA sequences from our loci (ITS, nuLSU, mtSSU, RPB1) were generated for this study (Table 1). The concatenated dataset had 3138 nucleotide positions. Molecular data supported the presence of 17 lineages of Astrothelium (Fig. 1), including five new species (A. flavocoronatum, A. macrostiolatum, A. neglectum, A. neovariolosum and A. siamense) and a new record from Thailand (see Taxonomic Treatment).
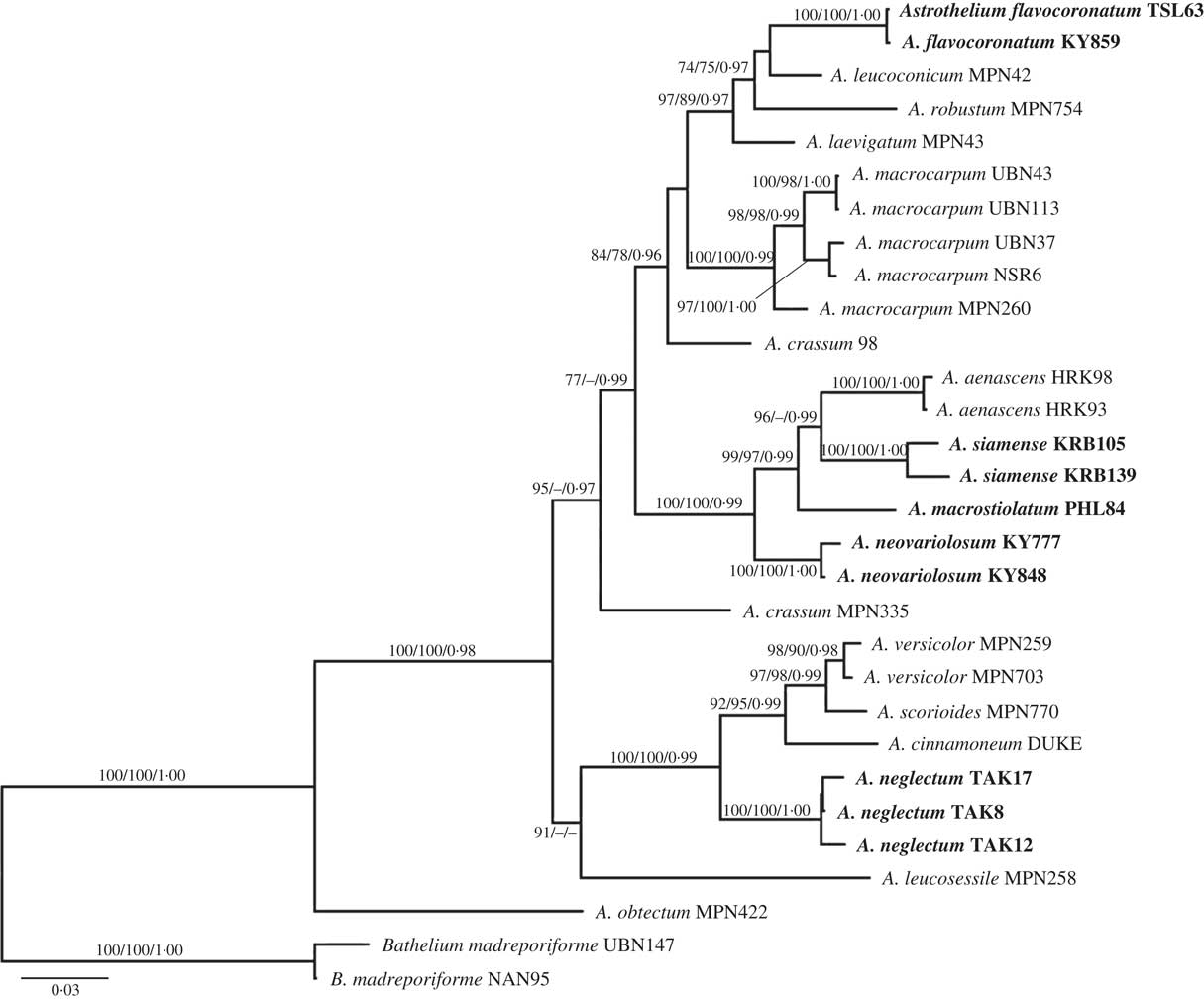
Fig. 1 Phylogenetic relationships of selected astrothelioid species in the genus Astrothelium based on maximum likelihood, maximum parsimony and Bayesian inference analyses using four loci (ITS, nuLSU, mtSSU, RPB1). The most likely tree obtained using RAxML is shown here. ML and MP bootstrap values ≥70% and posterior probabilities ≥0·95 are given at branches in this sequence.
The new species A. macrostiolatum, A. neovariolosum and A. siamense are closely related and also share certain phenotypic characters, such as a green thallus, white pseudostromata lacking anthraquinones and a hamathecium inspersed with oil droplets. However, they differ in ascospore characters (see below). These three new species form a paraphyletic grade basal to A. aenascens Aptroot (Fig. 1), which agrees in the inspersed hamathecium but differs in producing anthraquinones on ascomata. Astrothelium flavocoronatum also differs from the other new species in containing anthraquinones on the ascomata. This new species is similar to A. aenascens Aptroot and A. macrocarpum (Fée) Aptroot & Lücking (syn.: A. galbineum Kremp.) (Aptroot & Lücking Reference Aptroot and Lücking2016) in having anthraquinones and 3-septate ascospores of a similar size. However, our molecular data supported the distinction of A. flavocoronatum from A. aenascens and A. macrocarpum. The molecular data also support that Astrothelium neglectum, a fifth new species, is distinct from A. neovariolosum and A. siamense, two species that are similar to the new taxon in having a green thallus, white pseudostromata and containing lichexanthone. However, ascospore characters differ between these taxa and the hamathecium lacks oil droplets in the latter two species.
Astrothelium macrocarpum (Fée) Aptroot & Lücking (syn.: A. galbineum Kremp.) has been reported as the most common Astrothelium species in Thailand (Vongshewarat Reference Vongshewarat2000). Specimens morphologically consistent with that species were also found in this study. However, although specimens from the Neotropics and Thailand form a monophyletic clade, the molecular data suggest that the Thai material is somewhat distinct from the neotropical material (Fig. 1). The circumscription of A. macrocarpum (as A. galbineum) has been discussed previously. Harris (Reference Harris1984) reduced A. ochrothelizum Müll. Arg. to synonymy with A. galbineum, while these two species were separated by Makhija & Patwardhan (Reference Makhija and Patwardhan1989) based on ascoma characters. In fact, in A. macrocarpum the ascomata are totally embedded in the pseudostromata as in the Thai material (Vongshewarat Reference Vongshewarat2000; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Aptroot Reference Aptroot2009).
Taxonomic Treatment
Astrothelium flavocoronatum Luangsuphabool, Aptroot & Sangvichien. sp. nov.
MycoBank No.: MB 816951
Similar to Astrothelium diplocarpum in having anthraquinone pigments around the ostiole neck, but differing in having smaller ascospores; thallus yellow to green, perithecial wall carbonized, ostiole with yellow anthraquinone, ascospores 3-septate, 22–28×8·0–9·5 µm.
Type: Thailand, Nakhon Nayok Province, Khao Yai National Park, montane evergreen forest, on tree bark, 14°26'N, 101°22'E, alt. 760 m, 2015, Luangsuphabool KY859 (RAMK-027890—holotype).
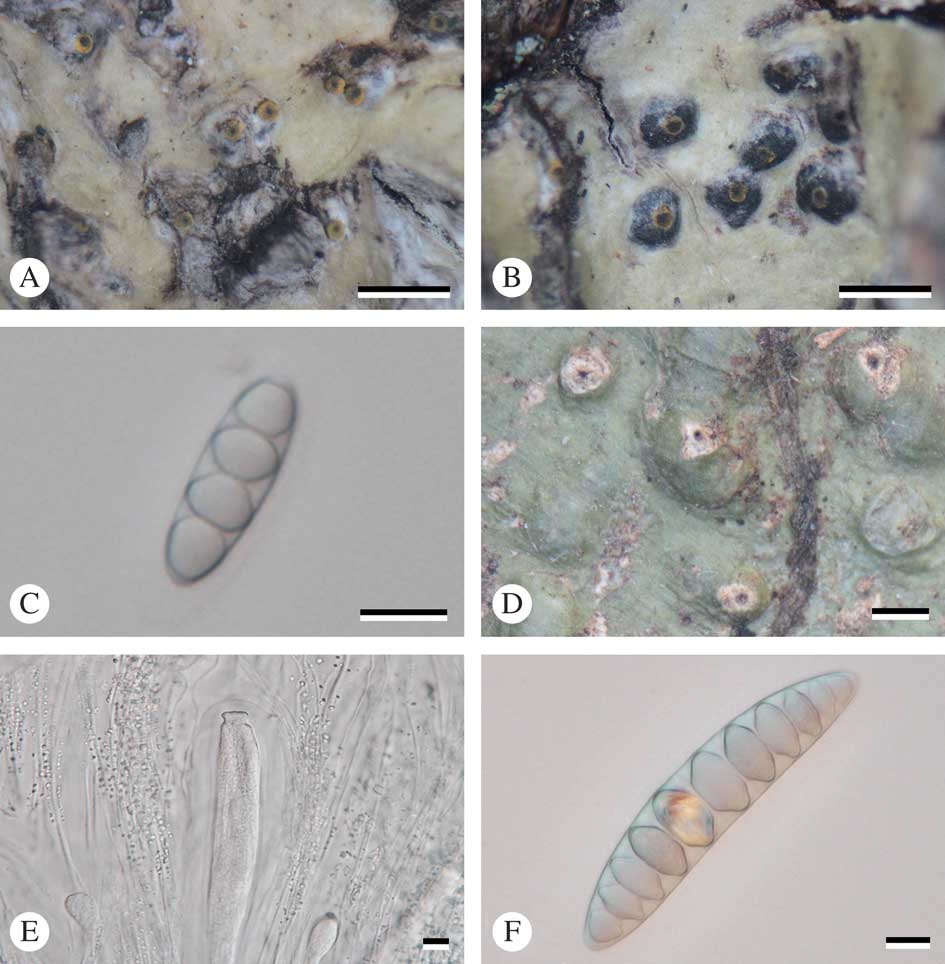
Fig. 2 A–C, Astrothelium flavocoronatum (holotype): A & B, thallus with ascomata; C, ascospores. D–F, Astrothelium macrostiolatum (holotype): D, thallus with ascomata; E, ascus and hamathecium inspersed; F, ascospores. Scales: A, B & D=1 mm; C, E & F=10 µm. In colour online.
Thallus crustose, corticate, yellow to green, smooth, continuous, prothallus black; cortex distinct, 40–70 μm thick; algal layer continuous, 35–75 μm thick; medulla 120–175 μm thick. Algae trentepohlioid.
Ascomata perithecia, pyriform, black, 0·40–0·85 mm diam., semi-immersed to emergent, solitary, usually consisting of two cavities that are joined with a common ostiole. Wall carbonized, ≤c. 50 µm thick. Ostiole apical, black, surrounded by yellow layer. Pseudostromata raised above the thallus, covered with thallus cortex or naked and carbonized. Hamathecium hyaline, not inspersed; paraphyses anastomosing, 0·85–1·00 μm thick. Asci clavate, 105–110×18·5–19·0 μm. Ascospores 8 per ascus, hyaline, transversely 3-septate, narrowly ellipsoid, 22–28×8·0–9·5 µm, lumina diamond-shaped to rounded.
Pycnidia not observed.
Chemistry. Thallus UV−, K+ yellow, C−, KC−, P−. Ascomata: around ostiole UV+ orange, K+ red, C+ red, P−. TLC: parietin, emodin.
Etymology. The specific epithet refers to the yellow tissue which surrounds the ostiole of the new species.
Notes. This new species is similar to the neotropical Astrothelium diplocarpum Nyl. in having anthraquinone pigments around the ostiole neck, but differs in having smaller ascospores (9-septate, 90–110×22–28 µm in A. diplocarpum) (Harris Reference Harris1995; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008). Also, A. macrocarpum (Fée) Aptroot & Lücking (syn.: A. galbineum Kremp.) and A. aenascens Aptroot are similar in having ascomata with anthraquinones and in the ascospore characters, but the new species differs in having ascomata with two locules (several locules with one to several ostioles in A. macrocarpum) and a non-inspersed hamathecium (inspersed in A. aenascens). Molecular evidence supports this distinction.
Additional specimen examined. Thailand: Phetchabun: Thung Salaeng Luang National Park, montane evergreen forest, on tree bark, 16°35'N, 100°52'E, alt. 740 m, 2008, Luangsuphabool TSL63 (RAMK-027889).
Astrothelium macrostiolatum Luangsuphabool, Aptroot & Sangvichien. sp. nov.
MycoBank No.: MB 816952
Similar to Astrothelium eustomum in thallus and pseudostroma characters, but differing in having 9–11 septate, 80–100×17–19 µm ascospores, an inspersed hamathecium and lacking secondary metabolites; thallus olive-green, pseudostroma with whitish ostiolar area, hamathecium inspersed with small oil droplets.
Type: Thailand, Loei Province, Phu Ruea District, Phu Luang Wildlife Sanctuary, montane evergreen forest, on tree bark, 17°16'N, 101°30'E, alt. 1460 m, 2014, Luangsuphabool PHL84 (RAMK-027895—holotype).
Thallus crustose, corticate, olive-green, smooth or somewhat warted, shiny, prothallus black; cortex distinct, 15–40 μm thick; algal layer continuous, 20–60 μm thick, medulla 25–90 μm thick. Alga trentepohlioid.
Ascomata perithecia, pyriform, black, 0·9–1·1 mm diam., common ostiole with two cavities, solitary or immersed in pseudostroma. Wall carbonized, ≤c. 100 µm thick. Ostiole apical, black. Pseudostromata white, mostly covered by thallus but leaving a large whitish ostiolar area free. Hamathecium hyaline, inspersed with small oil droplets usually less than 2 µm diam.; paraphyses anastomosing, 0·8–1·0 μm thick. Asci clavate, 240–300×37–55 μm. Ascospores 8 per ascus, hyaline, transversely 9–11 septate, fusiform, 80–100×17–19 µm, lumina diamond-shaped to rounded.
Pycnidia not observed
Chemistry. Thallus UV−, K+ yellow, C−, KC−, P−. Pseudostromata UV−, K−, C−, P−. TLC: no substances detected.
Etymology. The specific epithet refers to the large whitish ostiolar area.
Notes. This new species is similar to Astrothelium eustomum (Mont.) Müll. Arg. in thallus and pseudostromatal characters, and also to A. diplocarpoides Müll. Arg. and A. diplocarpum Nyl. in having rather large ascospores. However, it differs from those taxa in having more numerous septa, an inspersed hamathecium and a lack of secondary metabolites; 3–5-septate ascospores, a non-inspersed hamathecium, and lichexanthone are found in A. eustomum, 5–7-septate ascospores and lichexanthone are characteristic of A. diplocarpoides, and 9-septate ascospores, a non-inspersed hamathecium and anthraquinones are found in A. diplocarpum (Harris Reference Harris1984; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Lücking et al. Reference Lücking, Seavey, Common, Beeching, Breuss, Buck, Crane, Hodges, Hodkinson and Lay2011; Aptroot & Lücking Reference Aptroot and Lücking2016).
Astrothelium neglectum Luangsuphabool, Aptroot & Sangvichien. sp. nov.
MycoBank No.: MB 816953
Similar to Astrothelium eustomum in thallus, pseudostroma and ascospore characters, but differing by containing lichexanthone in the thallus; thallus yellow to green, pseudostromata white, hamathecium not inspersed, ascospores 3–5 septate, 21–25×7·5–9·5 µm.
Type: Thailand, Tak Province, Umphang District, Palatha Village, on tree bark, 15°49'N, 98°50'E, alt. 500 m, 2010, Luangsuphabool TAK17 (RAMK-027897—holotype).
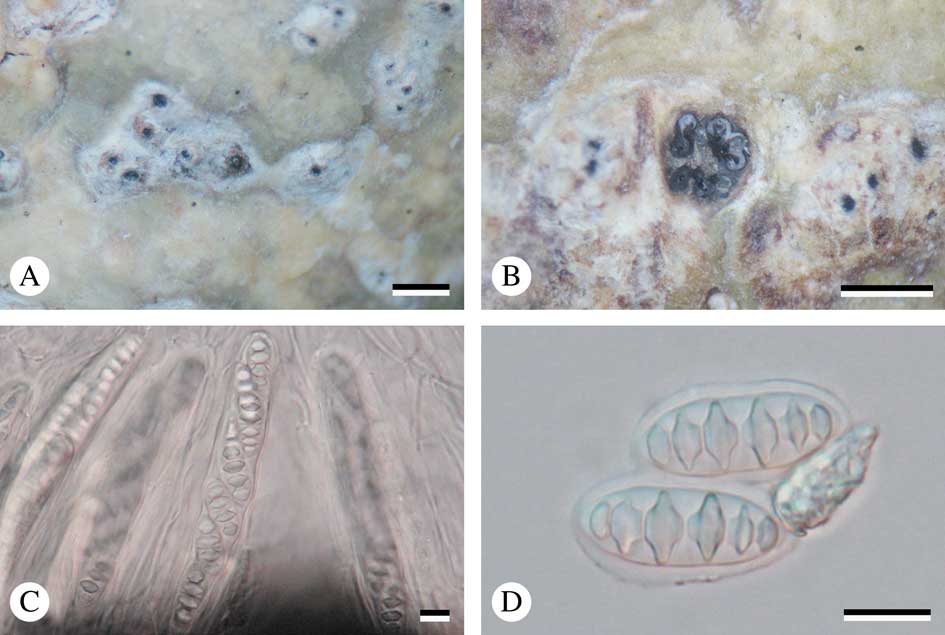
Fig. 3 Astrothelium neglectum (holotype). A, thallus with ascomata; B, ascomata; C, ascus with ascospores; D, mature ascospores. Scales: A & B=1 mm; C & D=10 µm. In colour online.
Thallus crustose, corticate, yellow to green, smooth, shiny, prothallus black; cortex distinct, 65–120 μm thick; algal layer continuous, 15–55 μm thick, medulla 60–165 μm thick. Alga trentepohlioid.
Ascomata perithecia, pyriform, black, 0·65–1·15 mm diam., 2–5 cavities with common ostiole immersed in pseudostroma. Wall carbonized, ≤c. 70 µm thick. Ostiole apical, black. Pseudostromata white, rounded to irregular, flattened top and raised above the thallus. Hamathecium hyaline, not inspersed; paraphyses anastomosing, 1·3–2·0 μm thick. Asci clavate, 110–140×15–20 μm. Ascospores 8 per ascus, hyaline, transversely 3–5 septate, fusiform, 21–25×7·5–9·5 µm, lumina diamond-shaped to rounded.
Pycnidia not observed.
Chemistry. Thallus UV+ yellow (lichexanthone), K+ yellow, C−, KC−, P−. Pseudostromata UV+ yellow (lichexanthone), K−, C−, P−. TLC: lichexanthone.
Etymology. The specific epithet refers to this species having been previously overlooked.
Notes. The new species is similar to Astrothelium eustomum (Mont.) Müll. Arg. in thallus, pseudostromatal and ascospore characters, but differs by containing lichexanthone in the thallus, whereas this substance is present only on the ostioles in A. eustomum (Harris Reference Harris1984; Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Aptroot Reference Aptroot2009). Also, A. neovariolosum and A. siamense are similar in having a corticated thallus, white pseudostromata, KOH− and lichexanthone, but the new species differs in ascospore characters and the non-inspersed hamathecium (3-septate ascospores, 17–23×6–7 µm, and hamathecium inspersed in A. neovariolosum; 4–7-septate, 30–50×10·5–12·0 µm, and hamathecium inspersed in A. siamense).
Additional specimens examined. Thailand: Tak: Umphang District, on tree bark, 15°49'N, 98°50'E, alt. 500 m, 2010, Luangsuphabool TAK8 (RAMK-027898), TAK12 (RAMK-027896).
Astrothelium neovariolosum Luangsuphabool, Aptroot & Sangvichien. sp. nov.
MycoBank No.: MB 816954
Similar to Astrothelium variolosum in pseudostroma and ascospore characters, but differing by an inspersed hamathecium; thallus greenish, pseudostromata grey to yellowish, ascospores 3-septate, 17–23×6–7 µm.
Type: Thailand, Nakhon Nayok Province, Khao Yai National Park, montane evergreen forest, on tree bark, 14°25'N, 101°22'E, alt. 750 m, 2013, Luangsuphabool KY777 (RAMK-027899—holotype).

Fig. 4 A–C, Astrothelium neovariolosum (holotype); A, thallus with ascomata; B, ascus with ascospores; C, ascospores. D–F, Astrothelium siamense (holotype); D, thallus with ascomata; E, ascus with ascospores; F, ascospores. Scales: A & D=1 mm; B, C, E & F=10 µm. In colour online.
Thallus crustose, corticate, greenish, smooth or somewhat warted, shiny, prothallus black; cortex distinct, 16–28 μm thick; algal layer continuous, 18–35 μm thick; medulla 40–85 μm thick. Alga trentepohlioid.
Ascomata perithecia, pyriform, black, 0·5–0·8 mm diam., fused ostiole with two cavities, single to 2–8 aggregate groups immersed in pseudostroma. Wall carbonized, ≤c. 50 µm thick. Ostiole apical, black. Pseudostromata grey to yellowish, raised above the thallus, round to irregular. Hamathecium hyaline, inspersed with oil droplets; paraphyses anastomosing, 0·9–1·0 μm thick. Asci clavate, 115–125×12·0–13·5 μm. Ascospores 8 per ascus, hyaline, transversely 3-septate, narrowly ellipsoid, 17–23×6–7 µm, lumina diamond-shaped to rounded.
Pycnidia not observed.
Chemistry. Thallus UV+ yellow (lichexanthone), K+ yellow, C−, KC−, P−. Pseudostromata UV+ brown-orange, K−, C−, P−. TLC: lichexanthone.
Etymology. The specific epithet refers to the morphological similarities with A. variolosum.
Notes. The new species is most similar to Astrothelium variolosum (Ach.) Müll. Arg. in having white to grey pseudostromata and in ascospore characters, but differs by its inspersed hamathecium (hamathecium not inspersed in A. variolosum) (Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Aptroot Reference Aptroot2009).
Additional specimen examined. Thailand: Nakhon Nayok: Khao Yai National Park, tree bark, 14°25'N, 101°22' E, alt. 760 m, 2014, Luangsuphabool KY848 (RAMK-027900).
Astrothelium siamense Luangsuphabool, Aptroot & Sangvichien. sp. nov.
MycoBank No.: MB 816955
Similar to Astrothelium variolosum in thallus and pseudostroma characters, but differing in having larger ascospores and an inspersed hamathecium; thallus olive-green to yellow, pseudostromata white, hamathecium inspersed, ascospores 4–7 septate, 30–50×10·5–12·0µm.
Type: Thailand, Krabi Province, Khlong Thom District, Thung Tieo-Sra Morakot trail, tropical rainforest, on tree bark, 7°55'N, 99°16'E, alt. 70 m, 2012, Luangsuphabool KRB139 (RAMK-027902—holotype).
Thallus crustose, corticate, olive-green to yellow, smooth, shiny, prothallus black; cortex distinct, 20–60 μm thick; algal layer continuous, 10–40 μm thick; medulla 10–40 μm thick. Alga trentepohlioid.
Ascomata perithecia, pyriform, black, 0·26–0·52 mm diam., common ostiole with two cavities, solitary to aggregated groups immersed in pseudostroma. Wall carbonized, ≤c. 30 µm thick. Ostiole apical, black. Pseudostromata white, raised above the thallus, round to irregular. Hamathecium hyaline, inspersed with oil droplets; paraphyses anastomosing, 0·7–1·0 μm thick. Asci clavate, 125–150×20–23 μm. Ascospores 8 per ascus, hyaline, transversely 4–7 septate, fusiform, 30–50×10·5–12·0 µm, lumina diamond-shaped to rounded.
Pycnidia not observed.
Chemistry. Thallus UV+ yellow (lichexanthone), K+ yellow, C−, KC−, P−. Pseudostromata UV−, K−, C−, P−. TLC: lichexanthone.
Etymology. The specific epithet refers to ‘Siam’, the traditional name for Thailand, where the species was collected.
Notes. This new species is similar to Astrothelium variolosum (Ach.) Müll. Arg. in having a green thallus and white to grey pseudostromata, but differs in having larger ascospores and an inspersed hamathecium (3-septate ascospores, 20–26×7–9 μm and hamathecium not inspersed in A. variolosum) (Aptroot et al. Reference Aptroot, Lücking, Sipman, Umaña and Chaves2008; Aptroot Reference Aptroot2009). The new species is also similar to A. neovariolosum in thallus and pseudostroma characters and in containing lichexanthone, but differs in having larger ascospores (3-septate, 17–23×6–7 µm in A. neovariolosum).
Additional specimen examined. Thailand: Krabi: Khlong Thom District, Hin Phoeng Waterfall, on tree bark, 7°51'N, 99°15'E, alt. 75 m, 2012, Luangsuphabool KRB105 (RAMK-027901).
New record for Thailand
Astrothelium aenascens Aptroot
Lichenologist 48: xx (2016).
Thallus crustose, corticated, greenish to grey, smooth. Alga trentepohlioid.
Ascomata perithecia, black, carbonized, aggregated groups immersed in pseudostroma and sharing common ostiole. Ostiole apical, black. Pseudostromata raised, containing yellow to orange pigment. Hamathecium hyaline, inspersed with oil droplets, branched and anastomosing. Ascospores 8 per ascus, transversely 3-septate, 24–30×9–10 µm, lumina diamond-shaped to rounded.
Chemistry. Thallus UV+ yellow to orange, K+ yellow. Pseudostromata UV+ red-orange, K+ red. TLC: lichexanthone, parietin.
Specimens examined. Thailand: Phitsanulok Province: Nakhon Thai District, Phu Hin Rong Kla National Park, montane evergreen forest, on tree bark, 16°59'N, 100°59'E, alt. 1310 m, 2009, Luangsuphabool HRK93 (RAMK-027887), HRK98 (RAMK-027888).
This study was financially supported by the Royal Thai Government through Ramkhamhaeng University, a CU Graduate School Thesis Grant and a scholarship from the Human Resource Development in Science Project (Science Achievement Scholarship of Thailand). We would like to thank Kansri Boonpragob and Kajonsak Vongshewarat for their helpful suggestions and Anupan Kongbangkerd and Thanakorn Wongsa for their assistance during fieldwork.









