Introduction
The torpedo rays of the genus Tetronarce Gill, 1862 are marine batoids distributed in tropical and temperate waters of the Atlantic, Indian and Pacific oceans (Last et al., Reference Last, White, de Carvalho, Séret, Stehmann and Naylor2016). Species of this genus found primarily in temperate waters have also been recorded in warmer seas (López & Bussing, Reference López and Bussing1982; Escobar-Sánchez et al., Reference Escobar-Sánchez, Galván-Magaña and Moreno-Sánchez2010; Morales-Serna et al., Reference Morales-Serna, Crow, Montes and González2019). Four of the 8 currently recognized species (Last et al., Reference Last, White, de Carvalho, Séret, Stehmann and Naylor2016) inhabit the Pacific Ocean: the Pacific torpedo, Tetronarce californica (Ayres, Reference Ayres1855) found on both sides of the Temperate North Pacific Ocean, the longtail torpedo, Tetronarce tokionis (Tanaka, Reference Tanaka1908) and the Taiwanese torpedo, Tetronarce formosa (Haas & Ebert, Reference Haas and Ebert2006) in the Western Pacific Ocean, and the Chilean torpedo, Tetronarce tremens (de Buen, 1959) in the South-eastern Pacific Ocean and part of the Tropical Eastern Pacific (Chile to Costa Rica). Species of Tetronarce are demersal and pelagic along the outer continental and insular shelves (Ebert et al., Reference Ebert, Haas and de Carvalho2015), with seasonal use of coastal waters (Dunton et al., Reference Dunton, Sparta, Frisk, Martinez and Shipley2021). Torpedo rays feed on bony fishes and small sharks (Parin & Kotlyar, Reference Parin and Kotlyar1985; Ebert et al., Reference Ebert, Haas and de Carvalho2015). These rays are occasionally captured beyond the shelf at mesopelagic depths (Parin & Kotlyar, Reference Parin and Kotlyar1985; Ebert et al., Reference Ebert, Haas and de Carvalho2015; Morales-Serna et al., Reference Morales-Serna, Crow, Montes and González2019).
Atypical records of vertebrates beyond their native distribution ranges in South America have recently occurred in Oaxaca, Mexico (Villegas-Zurita et al., Reference Villegas-Zurita, Elorriaga-Verplancken and Castillejos-Moguel2016; Tamayo-Millán et al., Reference Tamayo-Millán, Ahumada-Sempoal, Cortés-Gómez, Chacón-Romo Leroux, Bermúdez-Díaz and Islas-Villanueva2021). Here, we report the first finding of the Chilean torpedo Tetronarce tremens in the Mexican Pacific, based on a single specimen captured by artisanal fishermen off the coast of Oaxaca. In addition, we provide a phylogenetic analysis of Tetronarce based on a newly generated sequence of the cytochrome oxidase subunit I (cox1) gene along with all previously available ones.
Materials and methods
Fieldwork
Artisanal fishermen captured a specimen of Tetronarce tremens on 9 August 2020 using a bottom-set gillnet operating overnight off San Agustinillo, Oaxaca, Southern Mexican Pacific (15°39′45″N 96°32′20″W). Morphological identification of the specimen was achieved by following keys by de Carvalho et al. (Reference De Carvalho, Last, Séret, Last, Naylor, Séret, White, de Carvalho and Stehmann2016). Total length (TL) and disc width (DW) in cm (±0.1), and weight in kg (±0.01) were measured in the field. A small subsample of muscle tissue was preserved in 96% ethanol for DNA extraction, and the specimen was later fixed with 10% formalin and preserved in 70% ethanol. The specimen was deposited at the National Fish Collection of Universidad Nacional Autónoma de México (CNPE-IBUNAM), with catalogue number 23846.
Laboratory procedures
Total DNA was extracted using an Invitrogen PureLink Genomic DNA Mini Kit (ThermoFisher Scientific, Pittsburgh, PA) following the manufacturer's protocol. A ~656 bp sequence of the cytochrome oxidase subunit I (cox1) gene was generated using metazoan primers LCO1490-HCO2198 (Folmer et al., Reference Folmer, Black, Hoeh, Lutz and Vrijenhoek1994) under the following conditions: initial denaturation at 94° (1 min), followed by 35 cycles of 92°C (30 s), 48°C (40 s) and 72°C (1 min). Sequencing reactions were conducted at the Laboratorio Nacional de la Biodiversidad (LANABIO) in an ABI PRISM 3730 sequencer (Applied Biosystems, Carlsbad, CA). Sequences were reconciled and edited in Geneious Pro v.5.1.7 (Biomatters Ltd, Auckland, New Zealand).
Molecular analyses
The obtained DNA sequence was queried in GenBank using the standard nucleotide BLAST (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990). DNA sequences of species of Tetronarce available in GenBank were analysed in a phylogenetic framework, with Torpedo torpedo and Torpedo marmorata as outgroups (Table 1). Sequence alignment was performed in MAFFT (Katoh et al., Reference Katoh, Rozewicki and Yamada2019), and a Maximum likelihood phylogenetic analysis was performed in MEGA11 (Tamura et al., Reference Tamura, Stecher and Kumar2021), with 1000 bootstrap replicates under the GTR model, as suggested by the PhyML platform (Guindon et al., Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010) using the Akaike Information Criterion.
Table 1. Accession numbers for the cytochrome oxidase subunit I sequences used in the phylogenetic analysis of Tetronarce.

The sequence generated in this study appears in bold.
Results
Description
Male, medium-sized torpedo ray, measuring 54 cm TL, 37 cm DW and weighing 2.43 kg (Figure 1). Plain brown upper surface, lacking black spots (Figure 1A); ventral surface white (Figure 1B); disc broadly circular, wider than long; snout anterior margin weakly convex, with a median protuberance; eyes smaller than spiracle length; mouth small and strongly arched; first dorsal fin broad and rounded at apex, second dorsal fin smaller and slanted, with an oval apex; caudal fin large, with both lobes tall and broad, rounded at apices.

Fig. 1. Tetronarce tremens (de Buen, 1959) captured off Oaxaca, Mexico. (A) Dorsal view; (B) ventral view. Scale bar: 10 cm.
Molecular analyses
The BLAST query revealed a 99.85% identity with sequences of Tetronarce tremens from Chile, confirming morphological identification. In addition, the phylogenetic analysis (Figure 2) grouped the sequence of T. tremens from Oaxaca with the rest of the sequences of this species from Chile. Tetronarce tremens is sister to T. californica from California (North-eastern Pacific Ocean), and in turn, both species are grouped with a clade containing Tetronarce sp. from India (Bineesh et al., Reference Bineesh, Gopalakrishnan, Akhilesh, Sajeela, Abdussamad, Pillai, Basheer, Jena and Ward2016) and T. tokionis from Japan (Western Pacific Ocean). Sequences of the great torpedo, Tetronarce nobiliana (Bonaparte, 1835), from the Indo-Pacific and Eastern Atlantic Ocean, and the Western Atlantic torpedo, Tetronarce occidentalis (Storer, 1843), from Canada (North-western Atlantic Ocean) are placed on a separate branch.
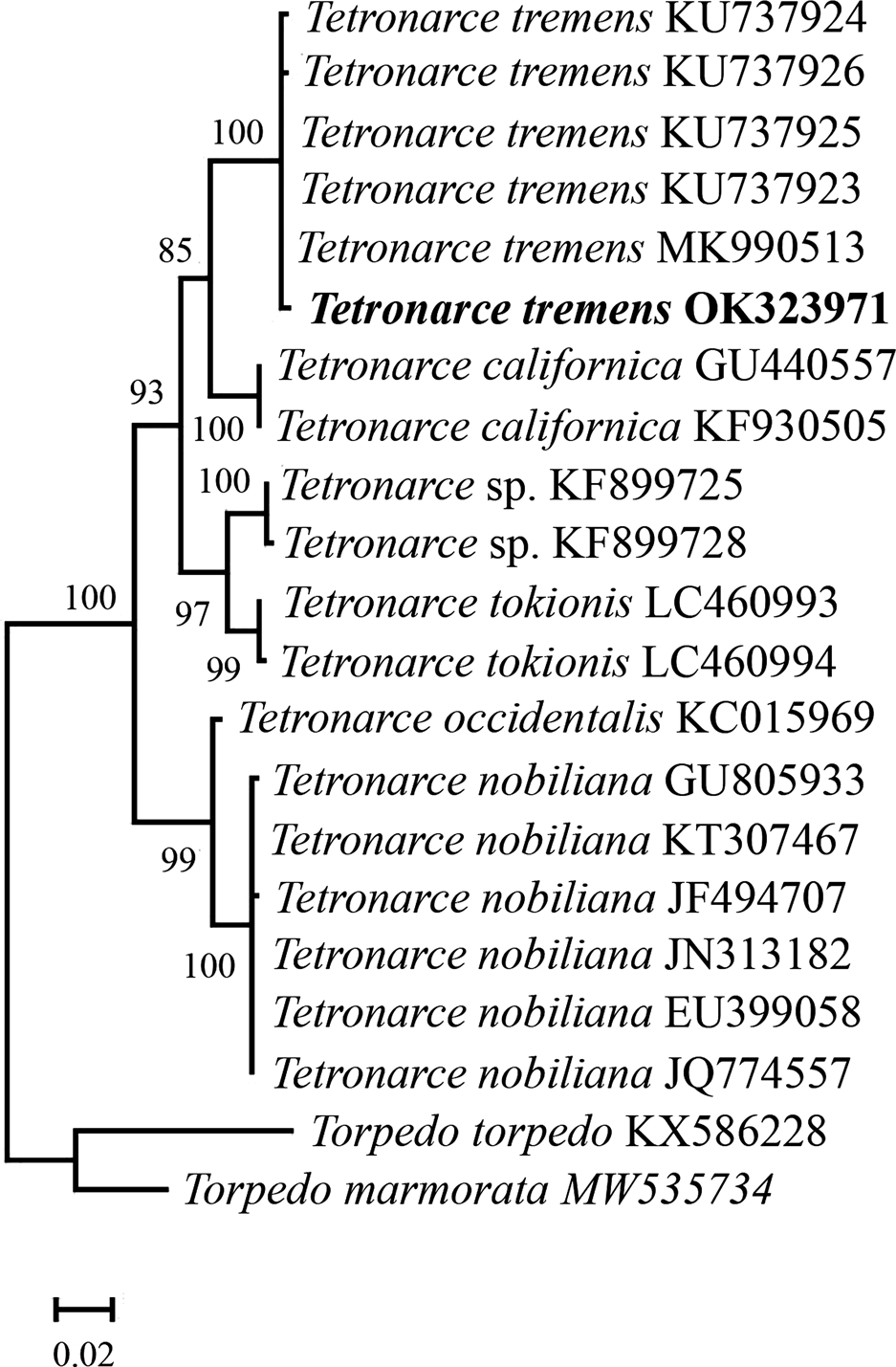
Fig. 2. Phylogeny of species of Tetronarce based on sequences of the cytochrome oxidase subunit I (cox1) gene.
Discussion
Three species of the order Torpediniformes occur along the Mexican Tropical Pacific: the Pacific dwarf numbfish, Diplobatis omnata (Jordan & Gilbert, 1890), the Cortez numbfish, Narcine entemedor Jordan & Starks, 1895, and the vermiculate numbfish, Narcine vermiculata Breder, 1928 (Last et al., Reference Last, White, de Carvalho, Séret, Stehmann and Naylor2016). The Chilean torpedo, Tetronarce tremens, is thought to be widely distributed in the Pacific Ocean (de Carvalho et al., Reference De Carvalho, Last, Séret, Last, Naylor, Séret, White, de Carvalho and Stehmann2016). Despite this, there are no previous records of this species in tropical waters north of Costa Rica, where it has been known to occur since the 1980s (López & Bussing, Reference López and Bussing1982). The absence of records in previous in-depth studies on batoids from the Mexican continental shelf (Torres-Huerta et al., Reference Torres-Huerta, López-Pérez, Tapia-García and Gracía2019), might indicate that no population of T. tremens inhabits the Mexican Pacific, in opposition to T. californica recently found in the Gulf of California (Escobar-Sánchez et al., Reference Escobar-Sánchez, Galván-Magaña and Moreno-Sánchez2010; Burgos-Vázquez et al., Reference Burgos-Vázquez, Cruz-Escalona and González-Acosta2019). Unverified sightings of ‘Torpedo nobiliana’ from Acapulco, Guerrero (Casassovici & Brosens, Reference Casassovici and Brosens2022) could belong to narcinid rays.
Climate change is expected to alter distribution ranges of marine species in the near future (Molinos et al., Reference Molinos, Halpern, Schoeman, Brown, Kiessling, Moore, Pandolfi, Poloczanska, Richardson and Burrows2016). Most notably, the distribution of Tetronarce tremens by 2050 is predicted to extend northwards to central Mexico, based on the IPCC RCP8.5 global warming scenario (Kaschner et al., Reference Kaschner, Kesner-Reyes, Garilao, Segschneider, Rius-Barile, Rees and Froese2019). Nevertheless, considering records of species of Tetronarce in the open ocean (Ebert et al., Reference Ebert, Haas and de Carvalho2015; Morales-Serna et al., Reference Morales-Serna, Crow, Montes and González2019), the specimen studied here could have been driven into shallow waters of the Mexican Tropical Pacific by favourable oceanographic processes (Tamayo-Millán et al., Reference Tamayo-Millán, Ahumada-Sempoal, Cortés-Gómez, Chacón-Romo Leroux, Bermúdez-Díaz and Islas-Villanueva2021).
Atypical sightings in the study area might be due to overlooked processes involving vertebrates in the open ocean off southern Mexico and Central America (Ballance et al., Reference Ballance, Pitman and Fiedler2006), as early empirical evidence of these occurrences can be traced back to the 1980s (Gallo-Reynoso & Ortega, Reference Gallo-Reynoso and Ortega1986). In light of the potential wide range of Tetronarce tremens in the Eastern Pacific Ocean, a deeper understanding of its foraging ecology would help assess the current record as an early indicator of climate-driven range shifts in the Tropical Eastern Pacific (Arvedlund, Reference Arvedlund2009; Fogarty et al., Reference Fogarty, Burrows, Pecl, Robinson and Poloczanska2017).
Acknowledgements
We thank Fidencio Spindola-Ávila (Cooperativa Pesquera Coyula, Puerto Ángel, Oaxaca) for cleverly noting this occurrence. Andrea Rebollo-Hernández, Nelly López-Ortiz, and Laura Márquez-Valdemar helped in the generation of the DNA sequence at Instituto de Biología, UNAM. Christian Lambarri-Martínez (Colección Nacional de Peces, Instituto de Biología, UNAM) provided the catalogue number for the specimen.
Author contributions
FR-E conceived the study. All authors analysed the data and wrote the manuscript.
Financial support
FR-E was supported by Consejo Nacional de Ciencia y Tecnología (CONACyT) scholarship 766341. The molecular sequence was obtained with funds from project PAPIIT-UNAM IN215722 to AO-F.
Conflict of interest
The authors declare none.
Ethical standards
All applicable international, national and/or institutional guidelines for use of animals were followed by the authors. The specimen was collected under elasmobranch fishing permits issued to fishermen of Cooperativa Pesquera Coyula, Puerto Ángel, Oaxaca, Mexico. The study is compliant with CBD and Nagoya protocols.
Data
The data generated during the current study are available in public repositories.





