Introduction
A brief account of the history of the Journal of the Marine Biological Association of the United Kingdom (JMBA) was published to coincide with 100 years since the publication of the first issue (Spooner et al., Reference Spooner, Howarth, Southward and Roberts1987). The present paper updates this account and reviews the influence that articles in the JMBA have had on disseminating the results of marine research. The selection of topics covered has had to be limited and the authors regret that it has not been possible to mention all the important papers published in the JMBA.
The Marine Biological Association of the United Kingdom (MBA) was conceived following debates, at the 1883 International Fisheries Exhibition in London, as to whether the major fisheries were inexhaustible or whether catches were already declining, in some areas, due to overfishing (Southward & Roberts, Reference Southward and Roberts1984). Prof. E. Ray Lankester, then at University College, London, appealed for the formation of a society to study marine life, including the habits and life-histories of food fishes. He organized an initial meeting at the Linnean Society in London to consider a proposal to establish a marine laboratory for the joint purpose of encouraging the study of marine zoology and making a scientific study of questions relating to sea fisheries (Lankester, Reference Lankester1887a, Reference Lankester1887b; Bourne, Reference Bourne1930). The MBA was formed at a meeting held at the Royal Society in London in March 1884. Construction of a laboratory in Plymouth commenced in February 1887, with the building opening the following year (Southward & Roberts, Reference Southward and Roberts1984).
Development of the journal
Remit and production of the JMBA
The JMBA was established in 1887, initially to inform members of the MBA of reports on the work of the Association and also of records of observations relating to the marine biology and fisheries of the coasts of the UK: fishermen and naturalists were invited to contribute (Lankester, Reference Lankester1887a). The first two numbers, of what is now called the ‘Old Series’ (OS), were published in August 1887 and August 1888. The first number contained a list of members of the MBA, an account of the formation of the Association and a description of the Laboratory, as well as an account of the local fishing grounds and fishing industry (see Figure 1; Heape, Reference Heape1887).
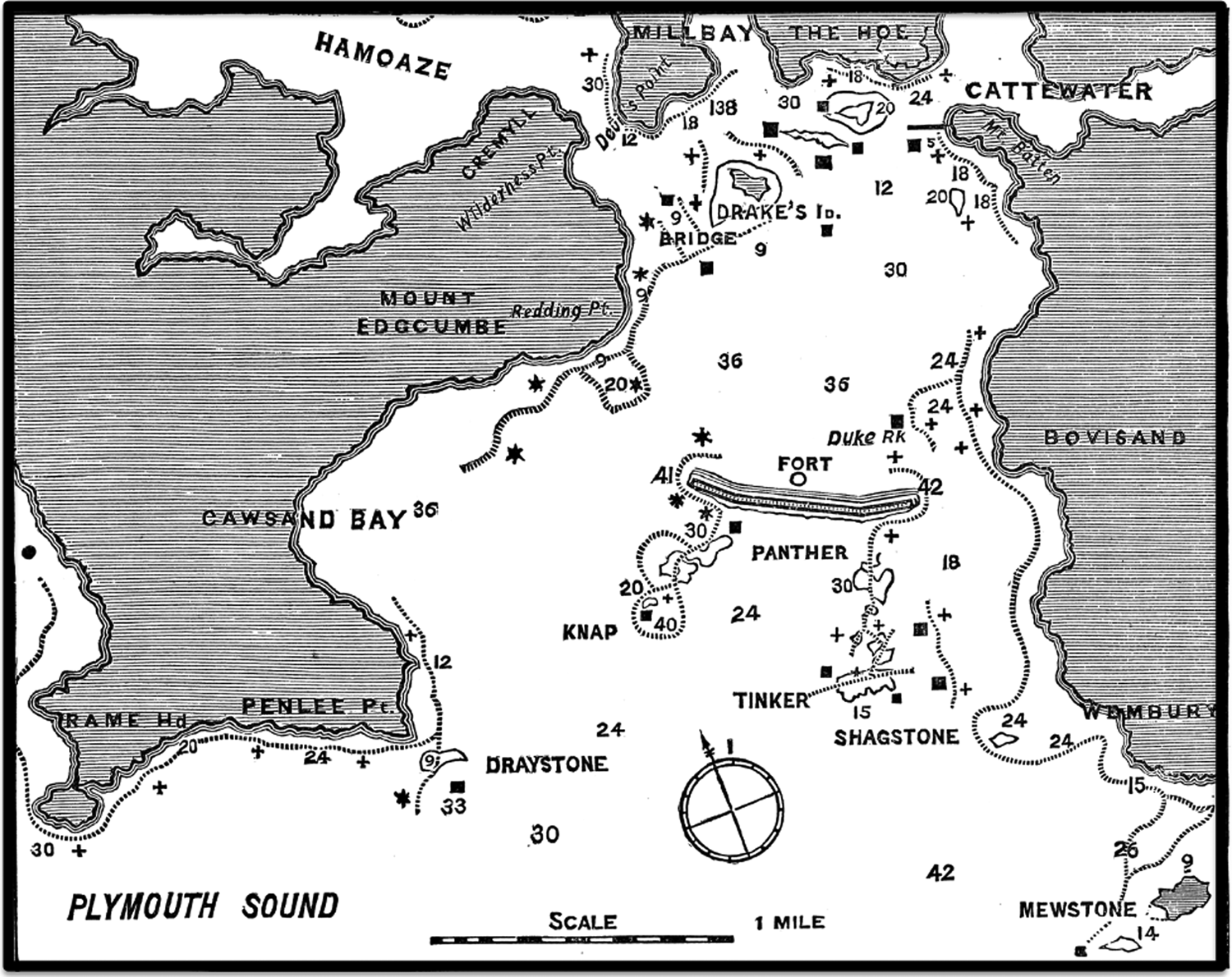
Fig. 1. From Heape (Reference Heape1887), original legend: ‘Fishing Map of Plymouth Sound, after map published in G. and R. Books ‘General Guide to Sea Fishing’ No. 5. The depth is marked in feet thus 30. The best places to fish for pollock, bass and mackerel are shown by the dotted line. The crosses show places to fish in ebb tide. The stars show places to fish in flood tide.’
G.C. Bourne, the first Director appointed, wrote that the JMBA was intended ‘to supply scientific information in an easily comprehensible form to those who are interested in marine fisheries’ as well as accounts of animal or vegetable morphology (Bourne, Reference Bourne1889). Bourne wrote ‘the Journal will be issued from time to time, according to the amount of work ready for publication, and will contain, besides the memoirs alluded to, abstracts of the scientific work done by the naturalists hiring tables in the Laboratory, notes and correspondence from other fishery and marine stations, abstracts of the most important results obtained by the fisheries commissioners of various Governments, and any correspondence addressed to the editor for publication’.
Since the early years, papers in the JMBA showed an appreciation of how the biota differed according to the origin of the water mass (Cleve, Reference Cleve1897). Papers were encouraged on ‘all aspects of marine biology and oceanography’. This broad remit continued until recent years when it was stated, in 1996, that ‘the primary topics covered are: (a) ecology, behaviour and fisheries; (b) physiology, ecotoxicology and biochemistry; (c) molecular, microbiology and genetics; (d) oceanography, satellite imagery and modelling’.
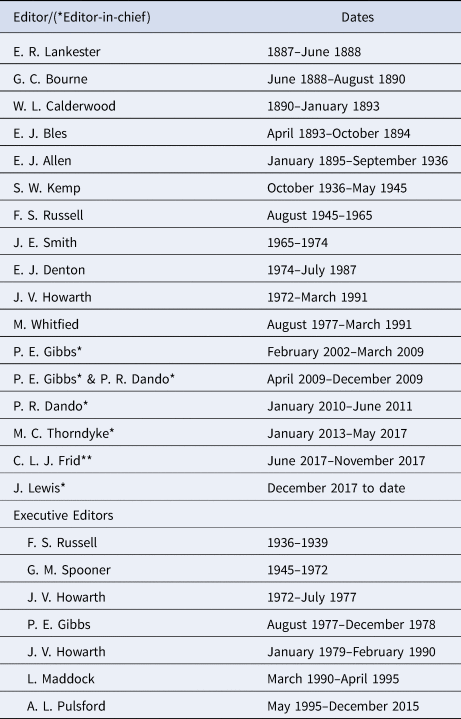
One of the duties of the Director under bye-law 14 was to ‘prepare and edit the Journal’ (Marine Biological Association, 1889). This duty was subsequently expanded in by-law 15 to ‘prepare and edit the publications of the Association'. This wording was continued in the Memorandum and Articles of the Marine Biological Association of the United Kingdom from 1927 and subsequent revisions in 1945, 1971 and 1995. With the increasing number of papers published in the JMBA it became impractical for the Director to edit the JMBA along with the other required duties. The 2003 revision of the Memorandum and Articles, by the then Director and Secretary, S.J. Hawkins, therefore changed the wording to ‘oversee the preparation and editing of the publications of the Association in liaison with the Editorial Board’. This wording is also used in the current Regulations and Rules of the Association, following the incorporation by Royal Charter in November 2017 (Marine Biological Association, 2017). As the editorial work increased, an Executive Editor was appointed to do a lot of the editorial work and subsequently the Director was replaced as Editor by an Editor-in-Chief who was responsible to the Director. A list of the editors, executive editors and Editors-in-Chief, with dates, is given in Table 1.
Table 1. Editors, Editors-in-chief* and Executive Editors of the JMBA since its formation (** Acting Editor)
Illustrations
The early illustrations in JMBA were drawings that were engraved onto printing plates and published as plates inserted following the individual articles. For example, Cunningham (Reference Cunningham1889) made camera lucida drawings, of fish eggs and larvae, that were converted into printing plates by Glyptographie Silvestre et Cie, Paris. In this early period, commercial cameras and photographic materials were being improved rapidly, making photography simpler and quicker for the amateur as well as the professional. Half-tone photographs and simple drawings were inserted into the text pages (Browne, Reference Browne1898) while more detailed drawings and collotype plates of photographs were inserted at the end of the paper as plates (e.g. Browne, Reference Browne1898, Reference Browne1907), as in other scientific publications of the period; an example is shown in Figure 2. Line drawings still remained the dominant mode of illustration. Marie Lebour, for example, published 67 papers on the planktonic larvae and biology of marine fishes and invertebrates in JMBA from 1916–1954 (available from the PLYMSEA repository), all beautifully illustrated with her own line drawings (e.g. Lebour, Reference Lebour1917, Reference Lebour1923, Reference Lebour1933, Reference Lebour1944). Her 1923 paper (Lebour, Reference Lebour1923) contains delicate drawings, within the text, of living medusae and Sagitta capturing fish larvae.

Fig. 2. Line drawing reproduced from Plate I in E.T. Browne (Reference Browne1907). Bimeria biscayana n. sp. Portion of a branch drawn to show the arrangement of the hydranths and the auxiliary tubes. [Renamed and re-described as: Amphinema biscayana (Browne Reference Browne1907) by Schuchert (2000).]
D.P. Wilson had been interested in photography from childhood. He took flash photos, using magnesium powder, of cuttlefish feeding in the aquarium (Wilson, Reference Wilson1946) and developed his own improved lighting systems for microphotography of living larvae and post-larvae, e.g. of the starfish Luidia (Wilson, Reference Wilson1978). From the mid 1950s papers using electron microscopy to describe microplankton and sub-cellular structures appeared in the JMBA. Parke and Manton started using the technique to study dinoflagellates (Parke et al., Reference Parke, Manton and Clarke1955). Over the following 50 years more than 130 papers in the JMBA, on a variety of topics, used electron microscopy in the studies reported.
From 1957 until 1985 the MBA employed G.A.W. Battin as a cartographer and draftsman. He standardized and improved the presentation and lettering of many of the figures published in the JMBA over this period (e.g. Newell, Reference Newell1967) and drew plans of the MBA Laboratory 1963 (Russell, Reference Russell1963).
Hand-coloured illustrations were occasionally used in earlier years (e.g. Parke Reference Parke1949) but colour photographs or computer diagrams were introduced regularly in the online version of papers from volume 82 (2002). Authors were given the option of paying for them in the printed issue, although coloured illustrations in the online versions were free. One problem was that some colour figures were converted to grey-scale for the printed version without checking that differences in the shading were clear.
Printing and appearance of the JMBA
The JMBA has been published in all except two years since 1887. The number of pages of scientific papers printed each year has steadily increased, with the exception of the war years, from <300 prior to 1904 to >500 in 1926 and 1927 and to >1700 since 2008 (Figure 3A). Initially, only one or two issues each year were published, with the exception of 1902 and 1905, when no issues were published and 1906 and 1930 which each had 3 issues. With the post-WWII expansion of marine research the number of issues a year was increased to 3 from 1951 and increased again to 4 from 1969 and to 6 from 1999. Eight issues a year, the current number, were printed, starting in 2008. Similarly the number of scientific papers published each year has increased from 6 in 1900 to over 170 today (Figure 3B).

Fig. 3. (A) Number of pages printed in the JMBA in each year since 1887. (B) Number of scientific papers published in the JMBA in the first year of each decade.
As the number of submissions increased, an editorial board was introduced to help in handling the submissions. By 1988 there was an editorial board of 12, drawn from eight countries. Currently the editorial board consists of eight individuals with an additional 34 associate editors drawn from 16 countries in total, reflecting the increased number of submissions and their broad international origin.
The journal was initially printed for the MBA by Adlard & Son, Bartholomew Close, London. This company continued printing the journal until 1897, volume 5, when the printing was transferred to William Brendon and Son, Mayflower Press, Plymouth. It was transferred again to Brook Crutchley, the printers for Cambridge University Press, in November 1937 (volume 22).
From the third number of the Journal, volume 1 of the New Series in 1889, the page size was increased to Royal Octavo to facilitate the preparation and appearance of illustrations. In November 1937 the page width was increased to allow for larger plates and figures. Following an increase in printing costs, the page margins were reduced to increase the amount of print on the page. The page width remained unchanged until February 2000, when the page size was increased to A4.
The Annual Council and Financial Reports for the MBA were published in the journal until 1992. From 1993 onwards they were issued as separate documents, freeing up more space in journal issues for scientific papers. The Annual Council Reports contained a summary of the work that scientists at the MBA had done during the year and contained advance publication of key results, as well as containing notes on observations on species and reports on scientific results that were not later published in the JMBA. For example, nearly all the neurophysiology studies conducted in the MBA Laboratory were published in the Journal of Physiology and elsewhere, although the key findings appeared in the Council Reports. In addition, abstracts of memoirs recording research carried out by researchers at the MBA Laboratory in Plymouth were printed in the JMBA from 1971 until 1980.
Changes in the appearance of the front cover of the journal over the years are illustrated in Figure 4. The original cover was pale blue, with an engraving of the MBA Laboratory, as seen from the sea (Figure 4A). In November 1937, with the transfer of publication to Cambridge University Press, the cover was revised and a new drawing of the MBA Laboratory was used on it. In June 1962, after enlargement of the Laboratory, a new engraving was used (Figure 4B). This continued to be used until 1989 when the cover changed to show a picture of waves breaking on a shore (Figure 4C). From 1999, volume 79, a picture of waves was inset into a black cover (Figure 4D). Coloured pictures were placed on the upper part of the front cover, first for special or themed issues, starting in February 2004 for an issue on ‘Biodiversity and distribution of species’ (Figure 4E) and from December 2005 onwards for every issue (Figure 4F).

Fig. 4. JMBA covers. (A) First issue (old series) cover; (B) cover from June 1962 showing the enlarged laboratory; (C) cover from 1989; (D) cover from 1999; (E) first coloured cover (February 2004): (F) typical cover from December 2005.
Typesetting for the JMBA was carried out at the MBA from the beginning of 1990 and continued until this was taken over by Cambridge University Press.
Online publication, archiving of papers and online submission
In the 1980s the librarians of the Marine Biological Association scanned the JMBA volumes, from the first issue in August 1887 to volume 40 (1961). The papers were made freely available on the library website as pdf files and these are now available through the PLYMSEA open access repository (http://plymsea.ac.uk/). Subsequently, when Cambridge University Press took over as publisher of the JMBA, these issues were rescanned in early 2009, as were subsequent issues to volume 78 (1998) and made available as the JMBA Archive on the publisher's website (https://www.cambridge.org/core/journals/journal-of-the-marine-biological-association-of-the-united-kingdom/all-issues). The JMBA archive was launched in May 2009 and then comprised over 65,000 pages, 5900 articles and almost 100,000 linked references. In the first year over 9000 pdf files were downloaded from the archive. Whereas papers in the archive on PLYMSEA can be freely downloaded as pdf files, the papers in the Cambridge University Press archive are only freely available, without payment, as title, authors and abstracts for those published post-1990. Most papers published pre-1990 show an extract, usually the first part of the Introduction, in place of the abstract, even if there is an abstract in the full paper.
JMBA papers were made available online, in the same month that the printed publication was issued, starting in August 1999. In 2008 papers started to be put online first, before printed publication.
In October 2005 the MBA set up a new online journal, JMBA 2 Biodiversity Records in response to the changing marine and coastal environment and an increasing demand for the documentation of marine organisms in locations where they had not formerly been recorded, as well as of species loss from habitats. Papers were published on the JMBA section of the MBA website. In 2008, Cambridge University Press took over this new journal, renaming it Marine Biodiversity Records (MBR) and hosting it on the Press website, making access free for subscribers to the JMBA. In January 2016 it was re-launched as the MBA's first online Gold Open Access Journal with Biomed Central (Springer-Nature) as the new publisher. The launch of these journals allowed more space in the JMBA for longer papers.
Online submission, using the ScholarOne system, was introduced for the JMBA (as well as for MBR) by Cambridge University Press in February 2009. The start did not go well. Manuscripts were returned to authors who had not submitted them via the new system, but had followed the, still-existing, instructions on both the MBA and Cambridge University Press websites. These stated that manuscripts should be sent as email attachments or copied onto a CD and posted.
In 2013 it was decided to make JMBA a ‘hybrid journal’ providing authors, upon acceptance of their article, with the option to pay an article processing charge for Gold Open Access publication. In 2018, 4.5% (10 of 222) scientific articles were open access, excluding editorials, forewords and corrigenda.
Reviews and special issues
Since the early years the JMBA has published occasional reviews. These were originally aimed at informing UK marine scientists of fishery practices, observations and research overseas, such as an account of the Newfoundland and Labrador fisheries (Grenfell, Reference Grenfell1894), fishery research in the USA (Cunningham, Reference Cunningham1894b), a report on flatfish research in Denmark (Stead, Reference Stead1896) and studies on the Norwegian fisheries (Garstang, Reference Garstang1897). Subsequently many reviews have been aimed at an international readership, e.g. on the distribution of medusae species across the oceans (Kramp, Reference Kramp1961), the photon flux required for photolithotrophs (Raven et al., Reference Raven, Kübler and Beardall2000), the worldwide migration of humpback whales (Rizzo & Schulle, Reference Rizzo and Schulle2009) and the reproduction and dispersal of invertebrates at vents and cold seeps (Tyler & Young, Reference Tyler and Young1999).
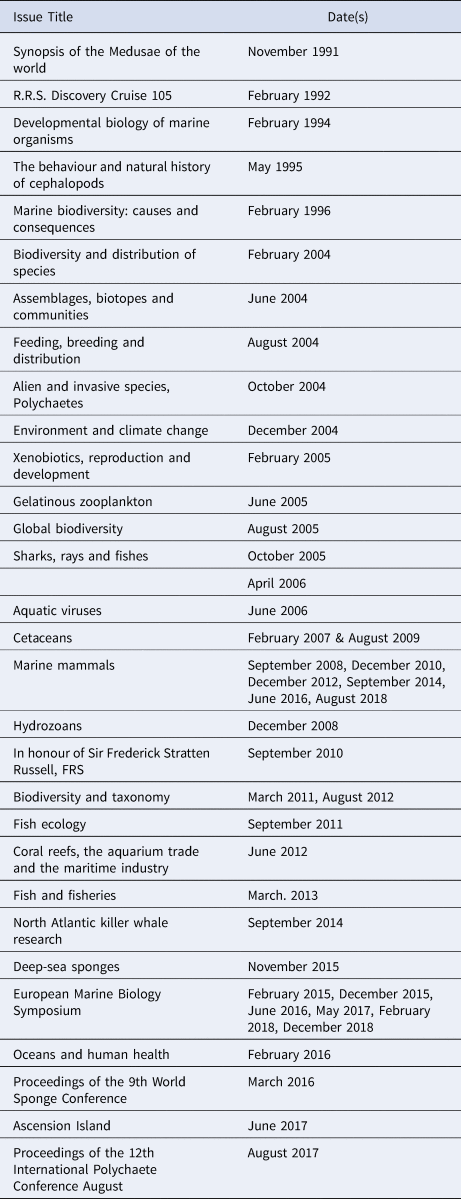
The JMBA has published a number of Special, or Themed issues and of issues, or issue sections, with Conference Papers (Table 2). Of particular note are the special issues, between 2007 and 2018, containing papers on cetaceans presented at annual meetings of the European Cetacean Society.
Table 2. List of Special/Themed issues and issue sections with Conference Papers in the JMBA
Three compilations have been published of all papers, including those in JMBA, of work that was done at the MBA. The first covered the years 1886–1913 (Anonymous, 1913), the second updated this to 1927 (Russell, Reference Russell1928) and the third covered the period 1928–1950 (Russell, Reference Russell1952).
The origin of published papers
Until about 1960, papers from the MBA staff and other scientists who carried out their research at the MBA, as well as members of the MBA, were prioritized for publication (Spooner et al., Reference Spooner, Howarth, Southward and Roberts1987). Since the 1960s the JMBA has been open to submissions globally and this is reflected in the increased numbers of papers (Figure 3B) as well as the change in the sources of published papers (Figure 5). Figure 5 allocates papers, published in the first year of each decade, as far as possible, to those carried out by MBA scientists, or by visiting scientists, at the MBA laboratories; those from the rest of the UK; those from Europe and those from other areas of the world. Papers were allocated to the region where most of the research was undertaken. Until the late 1960s, papers on work carried out at the MBA made up ~50% or more, of published papers. MBA scientists were then encouraged to publish in a wider range of journals and their submissions to JMBA sharply decreased. Subsequently, over 50% of papers published were from other UK laboratories until 2000 when papers from other European laboratories dominated publications in the journal. By 2010 the number of papers from South and Central America made up 30% of the total, reflecting the increase of marine laboratories and research activity in countries south of Mexico, with an increasing number also coming from Asia.
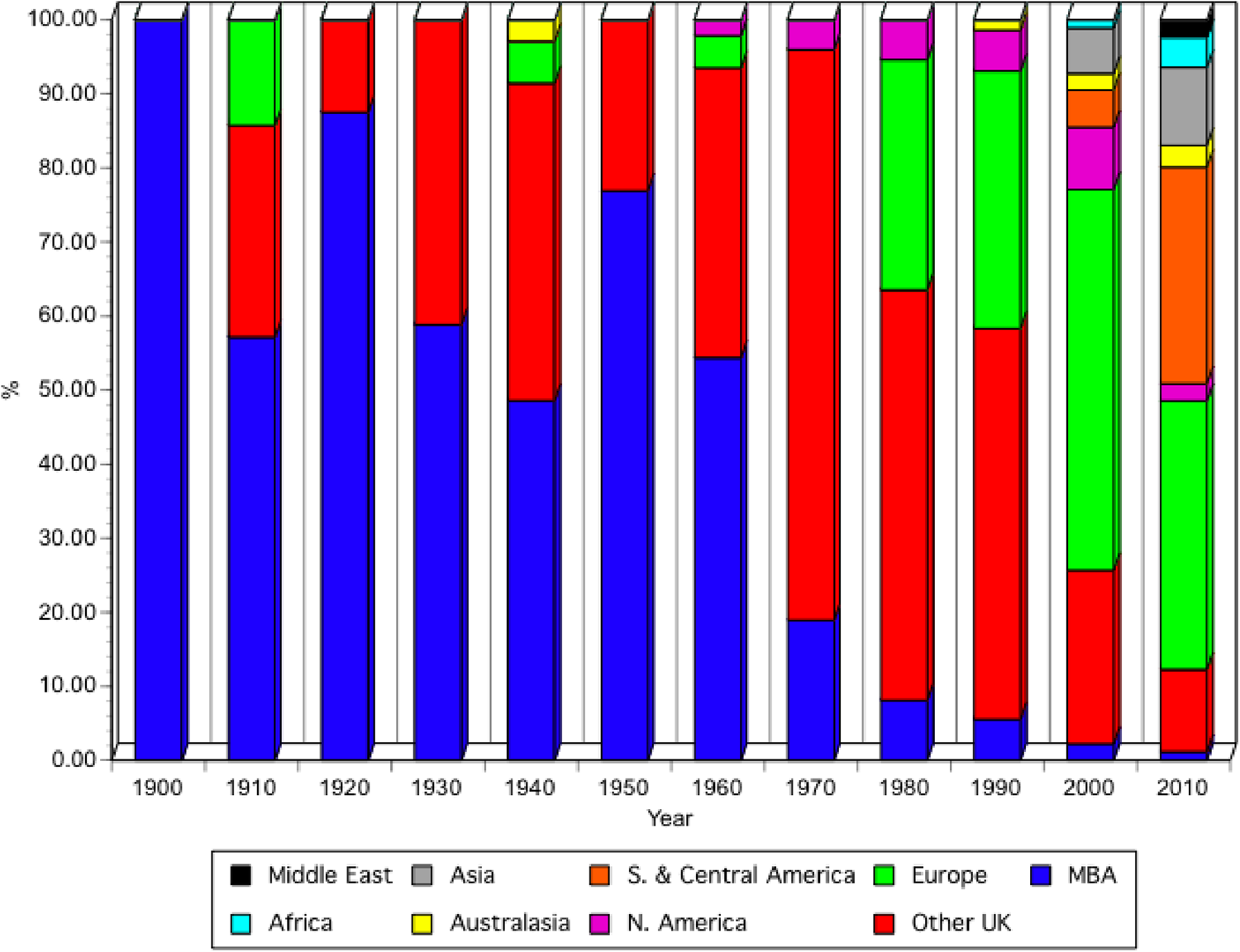
Fig. 5. Percentage of papers published in the JMBA, from the MBA, the rest of the UK or different regions of the world, in the first year of every decade, starting from 1900. North America includes Mexico.
Although the JMBA has not one of the highest citation rates of marine biology journals, currently with an impact factor of 1.578, it is one of only four with a citation half-life of >10 years (Fuseler-McDowell, Reference Fuseler-McDowell1988) and has kept this long citation half-life since the Science Citation Index started in 1964. This underlines the long-term value of so many of the publications in the JMBA. The 10 most cited papers in the JMBA during the first 60 years of publication are listed in Table 3 and the 10 most cited papers subsequently are listed in Table 4. In the first period, four of the 10 papers were on macroalgae whilst in the latter period four of the 10 were pollution-related.
Table 3. Top 10 citations in the Journal of the Marine Biological Association of the United Kingdom from 1887 to 1956
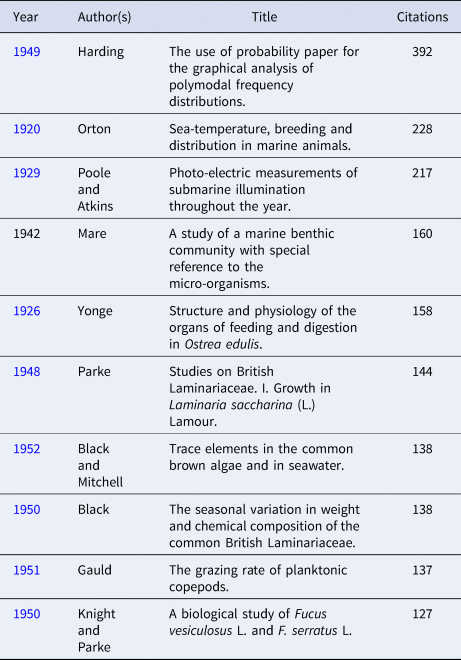
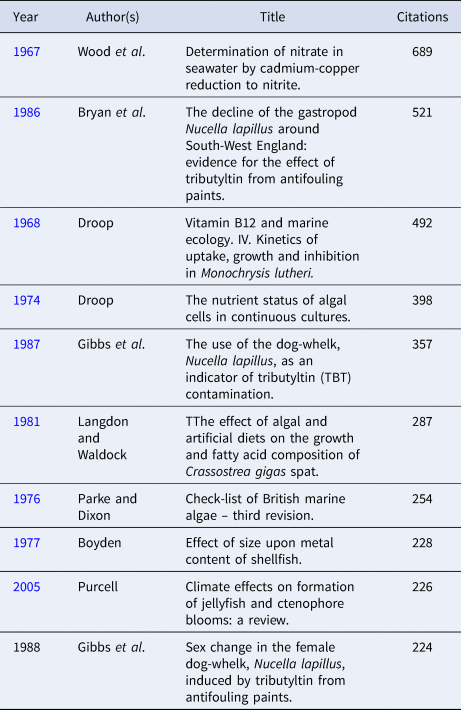
Table 4. Top 10 citations in the Journal of the Marine Biological Association of the United Kingdom from 1957 to present
The JMBA has been of particular use to the MBA Library (now the National Marine Biological Library) since copies have been exchanged for periodicals and annual reports from other institutions. This journal exchange was very important in the early years of the Library, but had declined to exchanges with 119 partners in 2014 and to 59 in 2019. The decrease being due to an increasing number of journals and reports becoming available online.
The influence of JMBA papers on marine research
Sampling methods and techniques
Studies on the distribution of marine organisms require reliable and repeatable sampling methods. Key equipment originally described in the JMBA included the improved plankton indicator (Hardy et al., Reference Hardy, Lucas, Henderson and Fraser1936) that developed into the continuous plankton recorder (Reid et al., Reference Reid, Colebrook, Matthews and Aiken2003). Plankton net tows at higher speed could be improved by fitting a front cone to reduce the entry area and improve filtration (Southward, Reference Southward1970). Studies on the vertical distribution of plankton (Russell, Reference Russell1926) required accurate depth-related sampling and stimulated the development of opening and closing mid-water nets (Russell, Reference Russell1925; Baker et al., Reference Baker, Clarke and Harris1973). A comparison of catches, made using nets of different mesh sizes, with those using a plankton pump were reported (Pyefinch, Reference Pyefinch1949), with the pump being considered best for quantitative sampling. Other net designs described included a neuston net (David, Reference David1965) and mid-water trawls with lights to attract fish and cephalopods (Clarke & Pascoe, Reference Clarke and Pascoe1985, Reference Clarke and Pascoe1998). Mobile deep-sea benthic carnivores can also be effectively observed using baited camera landers, with baited traps used to recover fauna in depths of >9000 m (Jamieson et al., Reference Jamieson, Lörz, Fujii and Priede2012).
Early attempts at enumerating bacterial numbers in seawater samples relied on culture techniques (Lloyd, Reference Lloyd1930). From 1970 onwards, epifluorescence techniques were used for direct counts, with acridine orange or DAPI as the fluorochrome (Turley & Hughes, Reference Turley and Hughes1994). Latterly, flow cytometry has been used to automate counts (Zubkov et al., Reference Zubkov, Allen and Fuchs2007).
For sampling benthos, new grabs were described for both gravel and mud bottoms (Smith & McIntyre, Reference Smith and McIntyre1954; Hunter & Simpson, Reference Hunter and Simpson1976). An adaptation of the Van Veen grab to fit a video camera allowed targeted sampling of the bottom (Mortensen et al., Reference Mortensen, Roberts and Sundt2000). New deep-sea benthic species were described from hauls taken by a modification of the early anchor dredge (Forster, Reference Forster1953). This was later developed into a heavier instrument that could dig-in, whichever side landed up (Holme, Reference Holme1961). Heavy, MBA-designed, anchor dredges were first used on the continental slope in the 1950s, resulting in the discovery of many new species, including Pogonophora, i.e. gutless frenulate siboglinid tube-worms (Southward & Southward, Reference Southward and Southward1958a).
Collection and observation of sub-littoral organisms by divers dates back to 1893, when Boutan (Reference Boutan1893) employed a diver to take underwater photographs. Lyle (Reference Lyle1929) published, in the JMBA, an account of seaweed distribution on the vertical faces of wrecks in Scapa Flow, using a professional diver to collect samples. Kitching et al. (Reference Kitching, Macan and Gilson1934) described a diving helmet, supplied with air pumped from the surface, that allowed a team of naturalists to survey the fauna of a sub-littoral gulley with near-vertical walls. A lack of insulated suits limited dives to ~15 minutes because of low water temperatures. Bainbridge (Reference Bainbridge1952) observed the behaviour of zooplankton, using a facemask and regulating valve with air fed from a compressed air cylinder on the shore or on a boat.
After WWII, self-contained underwater breathing apparatus (scuba) became more available and studies made with their aid started to be published in the JMBA in the 1950s. Forster (Reference Forster1954, Reference Forster1959) used scuba to descend to 24 m to observe the biota of rock faces and to study grazing by Echinus esculentus. Papers, recording specimens observed and collected by scuba diving, were also published on the distribution of sub-littoral algae around the Isle of Man (Kain, Reference Kain1960) and on the geology of the English Channel (Phillips, Reference Phillips1964).
Diver-operated equipment described include plankton nets (Potts, Reference Potts1976) and a diver controlled epibenthic dredge (Kritzler & Eidemiller, Reference Kritzler and Eidemiller1972). The use of a still-camera lander to take pictures along a transect for estimating overall epifaunal density was described (Vevers, Reference Vevers1951) and a stereo-camera system for close-up photographs of the seabed on the continental slope was also developed (Southward et al., Reference Southward, Robinson, Nicholson and Perry1976). McIntyre (Reference McIntyre1956) compared the efficiencies of the Agassiz trawl, Van Veen grab and still camera for estimating faunal densities on the same grounds. The use of towed video and still camera sleds (Holme & Barrett, Reference Holme and Barrett1977) allowed larger areas to be surveyed for epibenthos as well as by fitting a camera to the headline of a trawl (Dyer et al., Reference Dyer, Fry, Fry and Cranmer1982). For surveys at higher speed, an off-bottom towed camera was described (Blacker & Woodhead, Reference Blacker and Woodhead2009). Cameras and data loggers have also been fitted to large marine organisms to observe feeding behaviour, e.g. turtles (Heithaus et al., Reference Heithaus, McLash, Frid, Dill and Marshall2002).
In recent years, remotely operated vehicles (ROVs) have been used for both sampling (Ramirez-Llodra & Segonzac, Reference Ramirez-Llodra and Segonzac2006; Oliver et al., Reference Oliver, Vestheim, Antunes and Kaartvedt2015) and observations of organism behaviour e.g. in squid (Hunt et al., Reference Hunt, Zeidberg, Hamner and Robison2000) and leptomedusae (Hidaka-Umetsu & Lindsay, Reference Hidaka-Umetsu and Lindsay2018).
Biotope mapping on larger scales is now carried out acoustically, combined with ground-truthing by grabs, underwater video and/or still cameras and trawls (Brown et al., Reference Brown, Hewer, Meadows, Limpenny, Cooper and Rees2004; Foster-Smith et al., Reference Foster-Smith, Brown, Meadows and White2004). The development of this activity has become increasingly important with the establishment of networks of marine protected areas (MPAs) to ensure that they cover as much biodiversity as possible (Howell et al., Reference Howell, Davies and Narayanaswamy2010). A comparison of modern mapping techniques in the English Channel with previous methods described in the JMBA showed good correspondence (Coggan & Diesing, Reference Coggan and Diesing2008). For plankton blooms both airborne and satellite mapping techniques have been described (Garcia-Soto et al., Reference Garcia-Soto, Sinha and Pingree1996).
Many of these sampling methods and survey techniques, first published in the JMBA, are recorded in manuals on benthic sampling (Eleftheriou, Reference Eleftheriou2013), habitat mapping (Coggan et al., Reference Coggan, Populus, White, Sheehan, Fitzpatrick and Piel2007) and marine monitoring (Davies et al., Reference Davies, Baxter, Bradley, Connor, Khan, Murray, Sanderson, Turnbull and Vincent2001).
Molecular approaches to determine genetically discrete populations and cryptic species started with the use of electrophoretic techniques to study protein differences from the 1970s onwards, e.g. in barnacles (Dando & Southward, Reference Dando and Southward1980) and hydroids (Thorpe et al., Reference Thorpe, Ryland, Cornelius and Beardmore1992). From the 1990s PCR techniques allowed nucleic acid differences to be studied alone (Karageorgopoulos & Lewis, Reference Karageorgopoulos and Lewis2008) or together with protein differences (Wilding et al., Reference Wilding, Beaumont and Latchford1999).
Papers in the JMBA suggest that we can improve our knowledge of the past distribution of many species by DNA analysis of historical samples. Methods are now available to identify species in formalin-preserved Continuous Plankton Recorder samples, using PCR of extracted DNA (Kirby & Reid, Reference Kirby and Reid2001). This approach has been used to identify the main species responsible for the rise in echinoderm larval numbers in the North Sea from the late 1980s onwards, following a rise in sea temperatures, when larvae of Echinocardium cordatum dominated the plankton (Kirby & Lindley, Reference Kirby and Lindley2005).
Marking and tracking movements of marine fauna
Papers in the JMBA show the evolution of methods of marking marine animals to follow their movements. External tags, secured by ties through the muscle or skin of fish, or the epimeral suture of crabs (e.g. Hartley, Reference Hartley1947; Bennett & Brown, Reference Bennett and Brown1983), can be lost or loose tags can be placed on different specimens by fishermen wanting to claim the reward. Described methods to overcome this problem in fish include tattooing numbers on their opercula (Stevens, Reference Stevens1976) or by freeze-branding marks on their skin (Dando & Ling, Reference Dando and Ling1980). Branding has also been used to mark starfish (Kurihara, Reference Kurihara1998). Marking shelled molluscs by painting numbers or engraving marks on the shells has long been used. The technique has been applied to small juveniles using a code system of coloured dots (Gosselin, Reference Gosselin1993). A comparison of methods for marking scallops, Pecten maximus, concluded that flexible plastic tags fixed with cyanoacrylate gel was the preferred method (Ross et al., Reference Ross, Thorpe, Norton and Brand2001).
The problem with all the above methods is that the animal has to be recaptured to gain information. An advance came with the use of ultrasonic pingers that allowed the tagged individual to be detected underwater using either a directional hydrophone or a fixed array of omnidirectional hydrophones (Green & Wroblewski, Reference Green and Wroblewski2000; Giacalone et al., Reference Giacalone, D'Anna, Pipitone and Badalamenti2006). Ultrasonic pingers were used with pressure-sensitive transmitters to study the depth of eels in the water column during migration (Parker & McCleave, Reference Parker and McCleave1997). External data storage tags, recording time, temperature and pressure, allowed movements to be calculated after recovery of the tag and showed that movements of thornback rays were much greater than determined from the use of conventional tags alone (Hunter et al., Reference Hunter, Buckley, Stewart and Metcalfe2005). An improvement was made by inserting the tags in the peritoneal cavity to prevent tag loss (Righton et al., Reference Righton, Quayle, Hetherington and Burt2007).
Livestock ear tags have been used for marking blue sharks in mark–recapture studies (Stevens, Reference Stevens1976) but this method has now given way to geolocation tags for satellite tracking (Southall et al., Reference Southall, Sims, Metcalfe, Doyle, Fanshawe, Lacey, Shrimpton, Solandt and Speedie2003), with the advantage that boats are not needed to recover or track the fish. Satellite tracking has also been used successfully for turtles (Hays et al., Reference Hays, Webb, Hayes, Priede and French1991) and for following migrations and diving behaviour of cetaceans (Corkeron & Martin, Reference Corkeron and Martin2004; Eskesen et al., Reference Eskesen, Teilmann, Geertsen, Desportes, Riget, Dietz, Larsen and Siebert2009).
Microchemical analysis of otoliths is being increasingly used to show the habitats occupied by individuals at different stages during their lives, particularly with anadromous species. A recent example demonstrates that the majority of European eels, Anguilla anguilla, spend most of their adult lives in a particular salinity environment, without migrations away from this home range (Arai et al., Reference Arai, Kotake, Harrod, Morrissey and McCarthy2019).
Describing the marine ecosystem
A first task for the MBA, on establishment, was to record the species of marine animals and plants present locally, although much had been done by local naturalists prior to this (Boalch, Reference Boalch1996). In the first issue of the JMBA, the laboratory superintendent described the local fishing industry and the species caught (Heape, Reference Heape1887). Subsequently the fisheries for the main pelagic species, herring, pilchard and mackerel, were described (Ridge, Reference Ridge1889; Roach, Reference Roach1890; Calderwood, Reference Calderwood1891; Roach, Reference Roach1891) and research into the spawning and nursery areas, growth rates and fecundity of these species was reported (Ford, Reference Ford1933; Corbin, Reference Corbin1947; Steven, Reference Steven1949).
An initial fauna list for Plymouth Sound and the nearby offshore area was produced (Heape, Reference Heape1888). This formed the basis for assessing subsequent changes in the distribution of species. Additional lists for species groups, including sublittoral algae (Johnson, Reference Johnson1890), tunicates (Garstang, Reference Garstang1891), oligochaetes (Beddard, Reference Beddard1889), turbellaria (Gamble, Reference Gamble1893) and nemertines (Riches, Reference Riches1893) were published. A later publication described the meio- and micro-benthos and the methods of sampling these (Mare, Reference Mare1925). Seasonal changes in micro-plankton (organisms passing through fine nets) were described by Lebour (Reference Lebour1917). Baseline data on fish catches in South Devon bays (Garstang, Reference Garstang1903), a major study on the benthos and bottom deposits between Start Bay and the Eddystone (Allen, Reference Allen1899) and descriptions of the fauna of local estuaries (Allen & Todd, Reference Allen and Todd1900, Reference Allen and Todd1902; Percival, Reference Percival1929) were published. A detailed study of the distribution of the benthic fauna in the English Channel was undertaken by Holme (Reference Holme1966). Check-lists of British marine algae were published (Parke & Dixon, Reference Parke and Dixon1964, Reference Parke and Dixon1976) and a check-list of British marine diatoms was published in 1954 and updated in 1974 (Hendey, Reference Hendey1954, Reference Hendey1974). The latter list was later expanded to include freshwater and brackish water species (Hendey et al., Reference Hendey, Ross and Willims1986). As information on the local fauna accumulated separate fauna lists for the area were published in 1904, 1931 and 1957 (Marine Biological Association, 1904, 1931, 1957).
The breeding seasons, in the Plymouth area, of invertebrate species with planktonic larvae, were studied by identifying the larvae of species and recording their seasonal distribution in the plankton. Similarly, the breeding seasons of fish were established by examining the seasonal distributions of fish eggs and larvae in plankton hauls (Russell, Reference Russell1973). The papers in the JMBA on the identification of plankton have led to guides on the identification of species, e.g. Russell (Reference Russell1976) and Conway (Reference Conway2015).
Faunal studies on deep-sea species were first published on specimens collected in the Celtic Sea at depths down to 730 m (Bell, Reference Bell1890; Bourne, Reference Bourne1890) and in northern Biscay at depths down to 810 m (Browne, Reference Browne1907; Hickson, Reference Hickson1907; De Morgan, Reference De Morgan1913). More frequent collections were made in these areas from the 1950s onwards, with the increasing availability of suitable vessels, leading to the discovery of several new species, including sponges and bryozoans (Hayward & Ryland, Reference Hayward and Ryland1978), corals (Zibrowius, Reference Zibrowius1974), brachiopods (Atkins, Reference Atkins1959), polychaetes (Southward, Reference Southward1963) and fish (Forster, Reference Forster1967). In 2015 the JMBA published a special issue on the results of the International Workshop on Taxonomy of Atlanto-Mediterranean Deep-Sea Sponges (Xavier et al., Reference Xavier, Cárdenas, Cristobo, Van Soest and Rapp2015).
Since the 1990s there has been an increasing number of papers in the JMBA on the distribution, ecology and nutrition of deep-sea hydrothermal vent and cold seep fauna from sites worldwide, including the Atlantic (Gebruk et al., Reference Gebruk, Chevaldonné, Shank, Lutz and Vrijenhoek2000a; Stöhr & Segonzac, Reference Stöhr and Segonzac2005; Sitjà et al., Reference Sitjà, Maldonaldo, Farias and Rueda2019), the Caribbean (Gracia et al., Reference Gracia, Rangel-Buitrago and Sellanes2012), the Gulf of Mexico (Maldonaldo & Young, Reference Maldonaldo and Young1998), the Indian Ocean (Herring, Reference Herring2006), the South Pacific (Short & Metaxas, Reference Short and Metaxas2011), Japan (Yamaguchi et al., Reference Yamaguchi, Newman and Hashimoto2004) and the North Pacific (Southward et al., Reference Southward, Southward, Spiro, Rau and Tunnicliffe1994).
Nutritional studies and the food web
Important early JMBA papers were on the results of studies on the food web. Lebour published papers on the diet of post-larval fish (Lebour, Reference Lebour1918, Reference Lebour1919a, Reference Lebour1919b) and on the food of planktonic invertebrates, both from the stomach contents of freshly collected organisms and from observations on those given mixed plankton in plunger jars (Lebour, Reference Lebour1922, Reference Lebour1923). Food selection by juvenile fish was observed in aquaria (Lebour, Reference Lebour1919a). Much later, integrated studies on the plankton communities revealed the complex temporal relationships between viruses, bacteria, phytoplankton and zooplankton (Rodríguez et al., Reference Rodríguez, Fernández, Head and Harbour2000). Viruses were found to be a major controlling factor on the structure of both bacterioplankton (Hewson & Fuhrman, Reference Hewson and Fuhrman2006; Hewson et al., Reference Hewson, Winget, Williamson, Fuhrman and Wommack2006) and phytoplankton (Wilson et al., Reference Wilson, Tarran, Schroeder, Cox, Oke and Malin2002; Nagasaki et al., Reference Nagasaki, Tomaru, Shirai, Takao and Mizumoto2006) communities.
Early studies off Plymouth were reported on the food of benthic invertebrates from local fishing grounds (Hunt, Reference Hunt1925) as well as on the selection of benthic species as food items by different species of fish in relation to the quantitative abundance of their prey (Steven, Reference Steven1930). The latter study recognized the problems of organism avoidance of sampling gear and related the behaviour of both the bottom fauna and the fish to explain the observed species differences in diet. Both these publications used aquarium observations of the animals to help explain feeding differences between species. Observations on the behaviour of animals in aquaria were published both as collected notes, e.g. Wilson (Reference Wilson1949b) and as separate papers on individual species, including angler fish (Wilson, Reference Wilson1937), brachyurans (Lebour, Reference Lebour1944) and holothurians (Fankboner, Reference Fankboner1981).
The diets of the main pelagic fish in the area, herring, pilchard and mackerel, were described in JMBA papers. Bullen (Reference Bullen1912) found the diet of mackerel changed from the filtration of plankton to sight hunting of larger zooplankton and small fish during the season. Euphausids were a major component of the diet of herrings offshore (Lebour, Reference Lebour1924). In the estuaries herring mainly fed on mysids (Ford, Reference Ford1928b).
The identification of cephalopod species, using ‘beaks’ (Clarke, Reference Clarke1963) allowed the diets of apex predators to be described from stomach contents. The diets of large marine animals, described in JMBA papers, included those of giant squid (Lordan et al., Reference Lordan, Colins and Perales-Raya1998), albatross species (Seco et al., Reference Seco, Daneri, Ceia, Vieira, Hill and Xavier2016), blue sharks (Stevens, Reference Stevens1973), otters (Heggberget, Reference Heggberget1993), monk seals (Salman et al., Reference Salman, Bilecenoglu and Güçlüsoy2001) and whales (Lick & Piatkowski, Reference Lick and Piatkowski1998; Santos et al., Reference Santos, Pierce, Herman, Lopez, Guerra, Mente and Clarke2001). Some whales were found to eat a mixture of cephalopod prey or cephalopods and fish while others ate mainly a single cephalopod species (Clarke & Young, Reference Clarke and Young1998). These studies led to a review of niche separation in beaked whale species (MacLeod et al., Reference MacLeod, Santos and Pierce2003).
Since the beginning of this century there have been a number of papers in the JMBA on the use of stable isotope data to assess the trophic relationships of organisms including nematodes (Moens et al., Reference Moens, Bouillon and Gallucci2005), polychaetes (Nithart, Reference Nithart2000), gastropods (Korb, Reference Korb2003), vent shrimps (Gebruk et al., Reference Gebruk, Southward, Kennedy and Southward2000b) and crabs (Tsuchida et al., Reference Tsuchida, Suzuki, Fujiwara, Kawato, Uematsu, Yamanaka, Mizots and Yamamoto2011), ophiuroids (Fourgon et al., Reference Fourgon, Lepoint and Eeckhaut2006), sharks (Estrada et al., Reference Estrada, Rice, Lutcavage and Skomal2003), sea lions (Páez-Rosas & Aurioles-Gamboa, Reference Páez-Rosas and Aurioles-Gamboa2014) and whales (Mendes et al., Reference Mendes, Newton, Reid and Frantzis2007). These food web studies also demonstrate the wide geographic coverage of marine biology studies published in the JMBA, covering all the oceans.
Culture of marine organisms
Many papers in the JMBA describe attempts to culture marine phytoplankton, zooplankton, invertebrates and fish larvae. Early attempts were made to rear the larvae of food fishes in order to describe their developmental stages (Cunningham, Reference Cunningham1889, Reference Cunningham1894a, Reference Cunningham1894b; Garstang, Reference Garstang1900b; Jones, Reference Jones1972), although for the more common species it was possible to follow development stages from observations of the ichthyofauna in the plankton (e.g. Lebour, Reference Lebour1919c; Ford, Reference Ford1922; Demir, Reference Demir1972; Demir et al., Reference Demir, Southward and Dando1985).
These papers, as well as many others, contributed to Russell's identification guide to the eggs and planktonic stages of British marine fishes (Russell, Reference Russell1976).
A major advance was the development of the plunger jar system by E.T. Browne and the then MBA director, E.J. Allen (Browne, Reference Browne1898). A glass disc was moved up and down in an inverted bell jar full of seawater, by means of a water-filled bucket, that emptied via a siphon when full. The gentle movement of the water in the bell jar prevented the plankton sinking to the bottom of the vessel. This system allowed medusae and planktonic crustaceans to be kept alive and their behaviour, feeding, food preferences and development studied (Lebour, Reference Lebour1922, Reference Lebour1923). Similar observations were made on echinoderm (MacBride, Reference MacBride1900), fish (Lebour, Reference Lebour1925) and gastropod larvae (Lebour, Reference Lebour1933). The use of plunger jars also allowed planktonic larvae to be reared through metamorphosis so that their species could be identified, e.g. Lebour (Reference Lebour1934). These studies and others contributed to texts on embryology (MacBride, Reference MacBride1914; Young, Reference Young1990).
From the early issues, the JMBA has published papers on oyster cultivation, from accounts of it in Roman times (Günther, Reference Günther1897) and descriptions of oyster fisheries (Fowler, Reference Fowler1890) to experiments on rearing larvae to settlement on different algal cultures (Bruce et al., Reference Bruce, Knight and Parke1940). Feeding adult oysters on algal cultures before spawning was found to increase larval numbers and their survival (Helm et al., Reference Helm, Holland and Stephenson1973).
It was soon noted that phytoplankton required trace nutrients, such that artificial seawater media produced poor growth (Allen & Nelson, Reference Allen and Nelson1910; Allen, Reference Allen1914). Several species were found to require organic compounds for growth, reviewed by Johnston (Reference Johnston1955), including the haptophyte Isochrysis galbana Parke (Figure 6). Parke (Reference Parke1949) isolated the latter species from a fish pond. Subsequently high density culture techniques were developed for the species (Kain & Fogg, Reference Kain and Fogg1958), which required vitamin B12, as did the diatom Skeletonema costatum (Droop, Reference Droop1955). Isochrysis galbana is now the most widely cultured species for rearing bivalve larvae in aquaculture (Helm et al., Reference Helm, Bourne and Lovatelli2004) and S. costatum cultures are a good food for several bivalves and crustaceans including juvenile rock oysters (O'Connor et al., Reference O'Connor, Nell and Diemar1992) and shrimp post-larvae (Gleason & Wellington, Reference Gleason and Wellington1988). Cultures have been fed to adult Crassostea gigas infected by Alexandrium minutum, to speed up detoxification (Gueguen et al., Reference Gueguen, Bardouil, Baron, Lassus, Truquet, Massardier and Amzil2008).
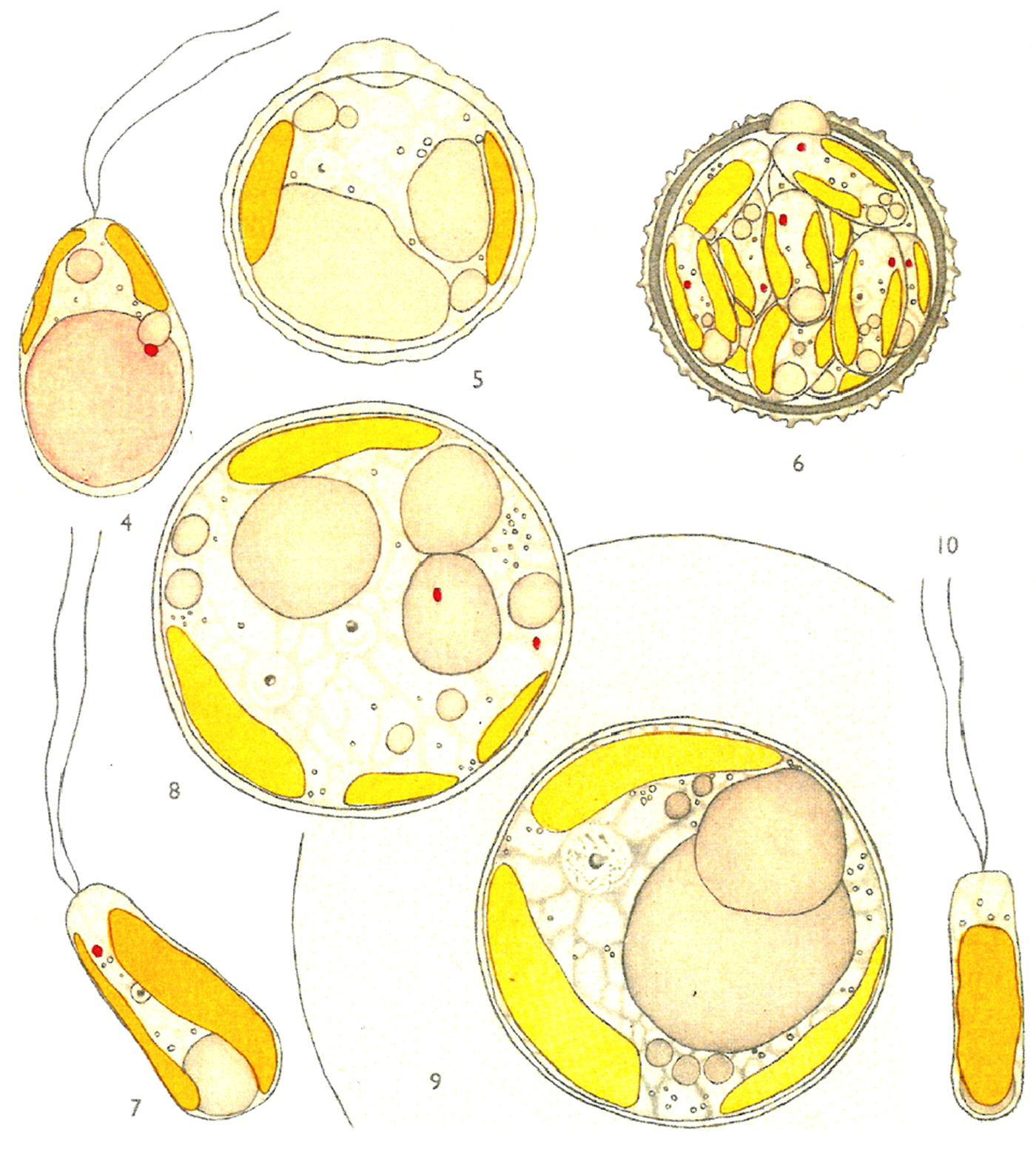
Fig. 6. Isochrysis galbana n.g. n.sp., reproduced from Plate 1 of Parke (Reference Parke1949), Figures 4–10, an example of an early hand-coloured illustration. 4, older motile stage; 5, immature cyst; 6, mature cyst; 7, young motile stage with lateral chromatophores; 8, reproduction in the palmelloid phase binucleate stage; 9, reproduction in the palmelloid phase, uninucleate stage; 10, young motile stage.
The rotifer Brachionus plicatilis Müller was also shown to require vitamin B12 for growth (Scott, Reference Scott1981). Brachionus plicatilis is a good first food for the post-larvae of the flatfish turbot and brill (Jones, Reference Jones1972). Studies on the diet of post-larval and young juvenile marine fish were reported (Lebour, Reference Lebour1918, Reference Lebour1919b, Reference Lebour1920) and have been widely cited, since they provided a basis for deciding the size and type of food organisms that were suitable for different fish species and at different stages of development, e.g. brill and turbot (Jones, Reference Jones1972) and pilchard (Blaxter, Reference Blaxter1969).
Allen & Nelson (Reference Allen and Nelson1910) described in detail methods for isolating single phytoplankton cells and keeping them in single species cultures. They listed 18 species of diatoms that they had in culture. Parke (Reference Parke1949) maintained six dinoflagellate species in culture at Plymouth, although two were lost as a result of wartime damage to the laboratory. These cultures formed the basis of the MBA Culture Collection, that now has ~400 strains (Marine Biological Association, 2018). Much of the MBA collection formed the basis of the Culture Collection of Algae and Protozoa when it was originally established in Cambridge, before being transferred to the Culture Collection of Algae and Protozoa at the Dunstaffnage Marine Laboratory. The MBA Culture Collection still holds strains of phytoplankton not in culture elsewhere and cultures, used for research and for larval food, are both supplied to, and deposited from, laboratories worldwide, e.g. Williams et al. (Reference Williams, Beckett and Maxwell2016), Taylor & Cunliffe (Reference Taylor and Cunliffe2017) and Xu et al. (Reference Xu, Ibrahim, Wosu, Ben-Amotz and Harvey2018).
Recording anthropogenic and environmental change
Overfishing
The 130 years since the JMBA was first published have seen many changes in the marine environment and in the distribution of species. Even by 1887 sewage pollution had depleted the number of fish species within Plymouth Sound (Bourne, Reference Bourne1889) and shellfish fisheries in the local estuaries had been badly affected by mine and china clay wastes (Anonymous, 1856; Heape, Reference Heape1887). One of the early papers considered the statistical evidence that overfishing had reduced stocks (Garstang, Reference Garstang1900a). The catch rates, of bottom fish, by vessels were compared, between 1875 and 1898, by converting the fishing power of steam trawlers to ‘smack units’, i.e. to the daily catch of a deep-sea sailing smack. Garstang concluded, ‘that the rate at which sea fishes multiply and grow, even in favourable seasons, is exceeded by the rate of capture’. Unfortunately these words were not heeded and the same unit-effort approach to fisheries statistics has shown a still continuing decline of North Sea fish stocks (Thurstan et al., Reference Thurstan, Brockington and Roberts2010).
Changes in temperature and other climate variables
The early JMBA publications, as well as many others, provided the initial basis from which the effects of both anthropogenic and climate change on the marine ecosystems in UK waters were subsequently detected. Both short-term changes, such as the effect on the benthos of the cold 1928–29 (Orton & Lewis, Reference Orton and Lewis1931) and 1962–63 winters (Holme, Reference Holme1967), and longer-term climatic changes (Southward, Reference Southward1960; Southward et al., Reference Southward, Hawkins and Burrows1995; Hawkins et al., Reference Hawkins, Southward and Genner2003; Capasso et al., Reference Capasso, Jenkins, Frost and Hinz2010; McHugh et al., Reference McHugh, Sims, Partridge and Genner2010; Mieszkowska et al., Reference Mieszkowska, Sugden, Firth and Hawkins2014) have been described, based to a greater or lesser degree on earlier studies published in the JMBA.
It was Cunningham (Reference Cunningham and Page1906), appointed in July 1887 as the first fisheries naturalist to the MBA, who first noted an alternation between warm-water species and cold-water species in the seas off south-west England, writing ‘As the Cornish coasts form the northern limit of the range of the pilchard, it seems possible that in certain periods the drift of warm water from the south extends further to the north, and that the pilchard then extends its wandering – while in other periods the drift of warm water is weaker or takes another direction, and that for this reason the north coast is deserted by the pilchard and visited by the herring’ (Cunningham, Reference Cunningham and Page1906). Orton (Reference Orton1920) noted that a minimum breeding temperature appeared to be a physiological constant for marine species and that this was one of the ways in which temperature controlled their distribution. Changes in mean seawater temperatures were shown to correlate well with an alternation between pilchard and herring fisheries (Southward et al., Reference Southward, Boalch and Maddock1988). Related changes between water temperatures and the distribution of other fish species in the region were reported (Stebbing et al., Reference Stebbing, Turk, Wheeler and Clarke2002). In the late 1920s the local herring fishery supported 300 vessels working out of Plymouth (Ford, Reference Ford1928a). This fishery collapsed shortly afterwards, during the 1930s (Southward et al., Reference Southward, Boalch and Maddock1988), due to a regime shift in the ecosystem in the northern North Atlantic resulting in warmer sea temperatures, leading to colder water species migrating northwards (Drinkwater, Reference Drinkwater2006).
Similar changes in the distribution of fish post-larvae, a series started by Russell (Reference Russell1930) and followed for almost 50 years (Russell, Reference Russell1973), showed long-term changes in species and species abundance that correlated with changing climate and water temperatures. Purcell (Reference Purcell2005) reviewed the literature on the distribution and abundance of jellyfish and ctenophore species and concluded that ocean warming was a cause of changes in their distributions.
Changes in zooplankton composition, as well as in phytoplankton and zooplankton abundance (Russell, Reference Russell1973; Robinson & Hunt, Reference Robinson and Hunt1986) correlated with environmental variables and climatic changes. The observed inter-annual variations in sea surface and seabed temperatures were considered to be due to changes in the atmospheric circulation patterns brought about by changes in the amount of heat received by the Earth from the sun (Maddock & Swann, Reference Maddock and Swann1970). Movements of water masses could be tracked by ‘indicator’ species of zooplankton, such as species of Parasagitta and Calanus (Russell, Reference Russell1935; Southward, Reference Southward1962a). Changes in sea surface temperatures in a 150-year time series from the Bay of Biscay and adjacent areas, were correlated with the Atlantic Multidecadal Oscillation indices and to changes in the zooplankton in the western English Channel (Garcia-Soto & Pingree, Reference Garcia-Soto and Pingree2012). Patterns in phytoplankton distribution were related to changes in weather patterns (Maddock et al., Reference Maddock, Harbour and Boalch1989).
Examinations of the distribution of plankton, benthic invertebrates and fish species require boats, nets and corers, grabs, or cameras, to assess species' occurrence and abundance. A less costly approach to study the effects of physical and chemical environmental changes on the distribution of marine species is to study the distribution of intertidal rocky shore organisms.
A classic, much-cited, early paper describing intertidal zonation at Wembury details the zonation of barnacles and macroalgae on rocks (Colman, Reference Colman1933). Moore (Reference Moore1936) subsequently examined the zonation distribution of the northern barnacle species Semibalanus balanoides and the southern barnacle Chthamalus stellatus around Plymouth, in relation to food supply and suspended matter in the water. This distribution study was extended to the British Isles and northern France (Moore & Kitching, Reference Moore and Kitching1939). Subsequently, Crisp & Southward (Reference Crisp and Southward1958) showed that changes in the relative distributions of the two species were correlated with their temperature tolerances (Southward, Reference Southward1958) and the environmental temperatures that influenced the beat rate of their cirri (Southward, Reference Southward1957, Reference Southward1962b). A longer-term, 40-year study showed that fluctuations in the relative numbers of Chthamalus and Semibalanus followed sea temperatures, with a 2-year lag, in a cyclical manner that was close to the 10–11 year sunspot cycle (Southward, Reference Southward1991). This pattern diverged from the solar cycle after 1975, probably related to major climatic changes (Southward, Reference Southward1991).
Distribution studies considering other intertidal species were reported by Crisp & Southward (Reference Crisp and Southward1958). The southern barnacle species Balanus perforatus was displaced from many sites by the exceptionally cold winter of 1962–63 but subsequently recovered and colonized sites as much as 100 km east of previous records for the English Channel (Herbert et al., Reference Herbert, Hawkins, Sheader and Southward2003). Studies of these distributional changes in the density and range of intertidal organisms were later expanded to cover the south-west peninsula and subsequently the whole British Isles and nearby Continental European shores. Fifty years of observations on the distribution and densities of intertidal fauna enabled links to be established between faunal changes in the eastern Atlantic intertidal zone and changes in the Atlantic Multidecadal Oscillation (AMO) (Mieszkowska et al., Reference Mieszkowska, Sugden, Firth and Hawkins2014). Historical datasets are proving vital for attempts to predict future changes in littoral ecosystems (Hawkins et al., Reference Hawkins, Mieszkowska, Firth, Bohn, Burrows, MacLean, Thompson, Chan, Little and Williams2015).
Changes in other environmental conditions
Papers in the JMBA describe the effects of a wide range of natural and anthropogenic effects on the marine ecosystem, including the effects of increased footfall over a rocky shore (Boalch et al., Reference Boalch, Holme, Jephson and Sidwell1974), changes in the benthos resulting from the discharge of china clay wastes (Probert, Reference Probert1975, Reference Probert1981) and the effects of eutrophication on infauna and fish (Quillien et al., Reference Quillien, Nordström, Le Bris, Bonsdorff and Grall2017). Both pre-effect and post-effect surveys have been used to study sites and comparisons of fauna changes along effect gradients used to evaluate impacts. One particularly interesting case was the impact of the infection of seagrasses, Zostera species, by a slime mould (Muehlstein et al., Reference Muehlstein, Porter and Short1991).
The fauna of the Salcombe estuary were first described by Allen & Todd (Reference Allen and Todd1900), including the species inhabiting the Zostera beds inside the harbour entrance. In the early 1930s Zostera beds started dying on both sides of the Atlantic (Graham & Atkins, Reference Graham and Atkins1938). Wilson (Reference Wilson1949a) examined the dead and dying Zostera marina beds near Salcombe that had been surveyed 50 years previously and found a marked decline in both the number of individuals and the numbers of macroinfaunal species, as well as marked changes in sand deposition due to the death of the Zostera. In the Tamar estuary, at Plymouth, Hartley (Reference Hartley1940), who studied the estuarine fish population in 1936 and 1937, wrote ‘in days gone by some of the Saltash men made a living all the year round by catching ‘smelts’ (Atherina presbyter). Now this fish has become so rare that its capture excites comment: I have myself seen only nine specimens in two years' work.’ This species spawns in Zostera beds (Kennedy & FitzMaurice, Reference Kennedy and FitzMaurice1969), attaching its eggs with long filaments to vegetation and its loss occurred at the time the Zostera beds died out. These studies have contributed to our knowledge of the role of Zostera beds in the marine ecosystem (Fonseca & Fisher, Reference Fonseca and Fisher1986).
Other major factors affecting the distribution of marine fauna include earthquakes. Although the 2016 Kaikoura quake is perhaps best known for its effects on marine life, no studies on the marine effects of this have been published to date in the JMBA, although changes in the zonation of intertidal biota following the 2011, Mw 9.0, earthquake in east Japan have been described (Noda et al., Reference Noda, Twasaki and Fukaya2016).
Marine chemistry
It was early appreciated that different water masses contained different plankton communities (Cleve, Reference Cleve1897). The chemical composition of seawater, not just the salinity, was related to the species composition, particularly of the phytoplankton (Atkins, Reference Atkins1923a, Reference Atkins1923b). Harvey (Reference Harvey1926), who devised a more accurate method for determining nitrate in seawater, by reduction to nitrite and colorimetric measurement of the nitrite after diazotization, using a Duboscq colorimeter, showed that uptake by phytoplankton removed all nitrate from the upper layers of the sea in summer. Nitrate was thought to be regenerated by vertical mixing with deeper water in winter but Pingree & Pennycuick (Reference Pingree and Pennycuick1975) showed that significant transfer also occurred through the thermocline. The analytical method for nitrate was refined by several authors but was always difficult to undertake at sea. Finally a more sensitive method was developed, passing water through copperized cadmium filings to convert the nitrate to nitrite (Wood et al., Reference Wood, Armstrong and Richards1967). This technique was subsequently adapted for automation, using AutoAnalyzer and submersible FIA systems (Oudot & Montel, Reference Oudot and Montel1988; Daniel et al., Reference Daniel, Birot, Blain, Tréguer, Leïldé and Menut1995; Zhang, Reference Zhang2000).
Papers in the JMBA also show developments in the measurement of dissolved phosphate in seawater. Early methods required large volumes of water and concentration of phosphate by precipitation, before colorimetric determination as phosphomolybdate (Matthew, Reference Matthew1916). The method was later refined and made more sensitive with a newly designed colorimeter (Harvey, Reference Harvey1948) and a single solution reagent for phosphate determination was subsequently introduced (Murphy & Riley, Reference Murphy and Riley1958). A comparative study of the methods of seawater inorganic phosphate analyses was made (Jones & Spencer, Reference Jones and Spencer1963). Atkins (Reference Atkins1923a) used the loss of phosphate from the water column during the year and the phosphate content of phytoplankton, to estimate the annual primary production of the English Channel as 1.4 kg phytoplankton m−2 over a water depth of 70 m. A similar production figure was obtained from the change in alkalinity of seawater due to photosynthesis (Atkins, Reference Atkins1922).
The complexity of seawater nutrient studies was revealed by the development of a method for determining total dissolved nitrogen and phosphorus by irradiating seawater with UV from a mercury arc lamp before analysis (Armstrong & Tibbitts, Reference Armstrong and Tibbitts1968). Analysis over an 11-year period showed that, unlike inorganic nitrogen and phosphate, total N and P changed little over the year (Butler et al., Reference Butler, Knox and Liddicoat1979). Thus the lost inorganic nutrients were replaced by equivalent organic nutrients that could be utilized by phytoplankton (Harvey, Reference Harvey1940; Antia et al., Reference Antia, Berland, Bonin and Maestrini1975).
Other significant analytical methods published in the JMBA include those for ammonia (Newell, Reference Newell1967), amino acids (Riley & Segar, Reference Riley and Segar1970), iron (Armstrong, Reference Armstrong1957) and silicate (Liss & Spencer, Reference Liss and Spencer1969). All the above, as well as other JMBA publications, helped to develop the methods described in later manuals of seawater analysis (e.g. Strickland & Parsons, Reference Strickland and Parsons1972; Grasshoff et al., Reference Grasshoff, Kremling and Ehrhardt1999).
Physical oceanography
In order to understand the movement of pelagic fish eggs it was necessary to understand the drift of surface water. Drift bottles, described by Nelson (Reference Nelson1922), that were weighted to make them almost completely submerged just below the surface or to float close to the bottom, were used to follow water movements throughout the year. Surface drift was measured in the English Channel. North Sea and Irish Sea (Garstang, Reference Garstang1898; Carruthers, Reference Carruthers1925). In subsequent studies surface and bottom drift bottles were deployed together (Carruthers, Reference Carruthers1927). Studies on water temperatures and salinities showed that episodic movements of surface water from the Atlantic into the English Channel occurred (Harvey, Reference Harvey1925). Carruthers et al. (Reference Carruthers, Lawford, Veley and Gruning1951) examined continuous current meter data from the Seven Stones Light Vessel, situated between Land's End and the Isles of Scilly, for 1939–1941 and found an ESE residual flow of 2.5 miles day−1. Deployment of a satellite-tracked ARGOS float, drogued at 10 m, south of the Isle of Scilly, revealed a residual clockwise current around the islands with a mean speed of 1 m s−1 in one circumnavigation (Pingree & Maddock, Reference Pingree and Maddock1985). Cooper (Reference Cooper1952a, Reference Cooper1952b) studied the effect of winds causing mixing of deep and surface water on the continental slope and the effect of canyons in channelling nutrient-enriched water into the English Channel by means of internal oceanic waves. He explained how similar conditions carried shoals of boar fish, Capros aper, well into the English Channel (Cooper, Reference Cooper1952a). In 1888 this deep-sea fish was filling the nets of trawlers working off Plymouth (Cunningham, Reference Cunningham1888).
Drogued ARGOS floats and neutrally buoyant ALACE floats, that rose to the surface at intervals to allow a satellite fix, were used to follow the path of a ‘meddy’ (an eddy with a core of high salinity Mediterranean outflow water) starting off the continental slope west of Lisbon (Pingree, Reference Pingree1995). The meddy was followed for 204 days with a mean westward track and had a rotational time of 2.5 days.
A compilation of oceanographic data from the above, and other, studies of the NE Atlantic (Pingree, Reference Pingree2002) revealed the complexities of the circulation system. A number of eddy systems were identified and followed, some of which had wave-like properties. The ARGOS and ALACE floats, together with current meter and shipboard measurements, allowed a correlation with satellite altimeter data of sea level anomalies, such that the altimeter data interpretation of eddies, internal wave and current systems could be verified. Long-term changes from 1992 to 2002 in the North Atlantic Current and the Subtropical Gyre transport were correlated with the winter NAO Index.
On a smaller scale, a study of wave-induced water circulation in sands (Webb & Theodor, Reference Webb and Theodor1972) described how dissolved and finely particulate organic matter would be supplied to interstitial microbiota (Meadows & Anderson, Reference Meadows and Anderson1968) and affect the small-scale distribution of epibenthic organisms.
Physiology and biochemistry
Although studies on the giant nerve axon of cephalopods are the best known of the physiological studies undertaken at the MBA (Sims, Reference Sims2014), the results were not published in the JMBA, but in journals including Nature and the Journal of Physiology. A summary of the history of this research was published in the JMBA (Hill, Reference Hill1950).
A theme of many physiology papers in the JMBA is how marine organisms can sense and respond to changes in the environment, such as temperature, pressure/sound/vibrations, salinity and light. A number of early papers describe the use of conditioned responses of fish to determine their ability to detect changes in temperature, to as little as 0.03°C (Bull, Reference Bull1936), salinity, vibrations (Bull, Reference Bull1928) and light wavelengths (Bull, Reference Bull1935). A mesopelagic deep-sea fish was shown to have different pigments in the part of the retina receiving light from above to the part receiving light from below (Denton & Locket, Reference Denton and Locket1989). Pioneering studies on bioluminescence and fish eyes were published in JMBA by J.A.C. Nicol in the 1950s. He first investigated the light-producing glands of the polychaete Chaetopterus (Nicol, Reference Nicol1952) and then luminescence in other marine species, with a much-cited joint paper with G.L. Clarke, on comparative studies on luminous pelagic animals, in JMBA (Clarke et al., Reference Clarke, Coer, David and Nicol1962).
In a series of papers on clupeids, the linked swimbladder-inner ear-lateral line system was described as the set of organs that could respond to vibrational pressures and allow the fish to estimate the direction and distance of the source (Allen et al., Reference Allen, Blaxter and Denton1976). The frequency responses of this system were described (Denton et al., Reference Denton, Gray and Blaxter1979; Gray & Denton, Reference Gray and Denton1979) as were the startle response of herring shoals (Blaxter & Hoss, Reference Blaxter and Hoss1981). The response was most sensitive in smaller fish with a threshold of 2–18 Pa (Blaxter & Hoss, Reference Blaxter and Hoss1981). Herring larvae only responded when the otic bulla contained gas, but showed a tactile response soon after hatching (Blaxter & Batty, Reference Blaxter and Batty1985).
Papers also describe how many marine organisms achieve neutral buoyancy in seawater. Many phytoplankton cells reduce their density, after sinking into dark nutrient-rich waters, so that they can ascend again into the photic zone for photosynthesis after acquiring more nutrients (Steele & Yentsch, Reference Steele and Yentsch1960). Pelagic eggs of teleostean fish achieve buoyancy by having a water content with a reduced salinity (Craik & Harvey, Reference Craik and Harvey1987). Lipid contributes only a minor component to the buoyancy.
Sodium and potassium ions were replaced by lighter ammonium ions in some squids to increase buoyancy (Clarke, Reference Clarke1979) and also in the chaeotognath Sagitta elegans (Bone et al., Reference Bone, Brownlee, Bryan, Burt, Dando, Liddicoat, Pulsford and Ryan1987). In some mesopelagic fish, neutral buoyancy was achieved by reducing skeletal density and protein mass (Denton & Marshall, Reference Denton and Marshall1958) and such fish are probably only capable of short burst swimming (Blaxter et al., Reference Blaxter, Wardle and Roberts1971). In contrast, most elasmobranchs reduce their density by depositing large amounts of oil in their livers (Bone & Roberts, Reference Bone and Roberts1969). The cephalopod Nautilus achieves buoyancy by replacing seawater in the shell chambers by gas and by water with a lower density (Denton & Gilpin-Brown, Reference Denton and Gilpin-Brown1966). Similarly Sepia achieves neutral buoyancy by displacing water with gas in the chambers of the cuttle bone (Denton & Gilpin-Brown, Reference Denton and Gilpin-Brown1961).
Clarke (Reference Clarke1978a, Reference Clarke1978b, Reference Clarke1978c), in three substantial papers in JMBA, provided evidence for the hypothesis that, because of the physical properties of spermaceti oil, sperm whales are able to achieve neutral buoyancy over their geographic range, when they dive below 200 m, by lowering the temperature of the oil in the spermaceti organ in their head by about 3°C. Clarke proposed that this was mainly done by cooling the blood, passing it through the skin in the head, before circulating around the organ.
A number of papers have reported mechanisms of camouflage in marine organisms, including the ways in which pelagic fish, such as silvery teleosts and hatchet fish, reduce their visibility to predators (Denton & Nicol, Reference Denton and Nicol1966; Janssen et al., Reference Janssen, Harbison and Craddock1986). The processes that create the silvery appearance during smoltification of salmon were also described (Denton & Saunders, Reference Denton and Saunders1972). An unusual elongated eye in the octopus Vitreledonella richardi was considered to minimize the silhouette and reduce predation (Land, Reference Land1992). Some sponges use an external sediment crust as camouflage (Schönberg, Reference Schönberg2016).
These adaptations of marine organisms to their environment now form part of the text of books on marine biology, marine ecology and the physiology of marine organisms (Newell, Reference Newell1976; Barnes & Hughes, Reference Barnes and Hughes1999; Kaiser et al., Reference Kaiser, Attrill, Jennings, Thomas, Barnes, Brierley, Hiddink, Kaartokallio, Polunin and Raffaelli2011).
The discovery of species of small, gutless, frenulate tubeworms (Southward & Southward, Reference Southward and Southward1958b; Southward, Reference Southward1978) led to investigations on their uptake of dissolved organic compounds from the sediment as a nutritional source (Southward & Southward, Reference Southward and Southward1980). Following the discovery of symbiotic, chemoautotrophic bacteria in their larger vestimentiferan relatives from hydrothermal vents (Cavanaugh et al., Reference Cavanaugh, Gardiner, Jones, Jannasch and Waterbury1981), it was discovered that similar bacteria also occurred in frenulates (Southward, Reference Southward1982; Southward et al., Reference Southward, Southward, Dando, Barrett and Ling1986). Further investigations showed that some thyasirid and lucinid bivalves (Dando & Southward, Reference Dando and Southward1986; Southward, Reference Southward1986) and nematodes (Austen et al., Reference Austen, Warwick and Ryan1993) also obtained nutrition from endosymbiotic chemosynthetic bacteria. Stable isotope studies revealed different nutritional sources for three chemosynthetic bivalve species at the Logatchev hydrothermal site (Southward et al., Reference Southward, Gebruk, Kennedy, Southward and Chevaldonné2001). Other species at vents can harvest chemosynthetic epibiotic bacteria from their surfaces (Suzuki et al., Reference Suzuki, Suzuki, Tsuchida, Takai, Southward, Newman and Yamaguchi2009; Tsuchida et al., Reference Tsuchida, Suzuki, Fujiwara, Kawato, Uematsu, Yamanaka, Mizots and Yamamoto2011).
These papers on bacterial symbionts in nematodes, siboglinid tubeworms, bivalves, crustaceans and other species have been cited in reviews of these fields (Kiel, Reference Kiel2010; Hilário et al., Reference Hilário, Capa, Dahlgren, Halanych, Little, Thornhill, Verna and Glover2011; Duperron et al., Reference Duperron, Gaudron, Rodrigues, Cunha, Decker and Olu2013).
Pollution studies
Oil spills
The massive oil spill resulting from the grounding of the Torrey Canyon oil tanker on the Seven Stones Reef, between Cornwall and the Isles of Scilly in 1967 led to a major investigation by the MBA into the effects of crude oil and of the oil dispersants used at that time on marine life. Publications showing the effects of oil and dispersants on organisms started appearing in the JMBA the following year (Corner et al., Reference Corner, Southward and Southward1968; Wilson, Reference Wilson1968; Bryan, Reference Bryan1969). These and other studies by scientists at the MBA resulted in the rapid publication of the book ‘‘Torrey Canyon' Pollution and Marine Life: A Report by the Plymouth Laboratory of the Marine Biological Association of the United Kingdom' (Smith, Reference Smith1968) that concluded that the oil dispersants used at the time were more damaging than the oil itself. This led to the JMBA becoming a popular journal for publishing studies on observations on the results of oil spills elsewhere, including the Amoco Cadiz spill off Brittany (Brule, Reference Brule1987), the Erika spill off Pays de la Loire (Pavillon et al., Reference Pavillon, Oudot, Dlugon and Roger2002), the Sea Empress spill off Wales (Reynolds et al., Reference Reynolds, Lancaster and Pawson2003), the Prestige oil spill off Spain (Bustamante et al., Reference Bustamante, Tajadura-Martin and Saiz-Salinas2010) and the Volgoneft-248 spill off Turkey (Tas et al., Reference Tas, Okus, Ünlu and Altiok2011).
Follow-up studies, on the recovery of the intertidal fauna in particular, were published, e.g. Southward & Southward (Reference Southward and Southward1978). Fifty years after the Torrey-Canyon spill the Industry Technical Advisory Committee (ITAC) annual meeting, organized by Oil Spill Response Ltd, was held in Plymouth, at which the problems of defining the long-term changes resulting from oil spills and dispersant use were summarized. A session was also included on the ecological and sociological long-term effects of the Torrey Canyon oil spill citing the early studies published in JMBA (Hawkins et al., Reference Hawkins, Evans, Moore, Whittington, Pack, Firth, Adams, Moore, Masterson-Algar, Mieszkowska and Southward2017b). The inter-tidal and low sub-tidal flora and fauna on some sheltered shores had taken 15 years to return to their pre-spill conditions and the value of long-term monitoring was emphasized. Oil spill dispersants have continued to be used in the marine environment, but the dispersant industry has modified the ingredients and now gives more attention to the ‘net environmental benefit’ of their use (Lessard & Demarco, Reference Lessard and Demarco2000).
Heavy metal pollution
Metal mining for lead, silver, tin and copper has an ancient history in Devon and Cornwall. Tin smelting dates back to the early Bronze Age (Miles, Reference Miles1975; Hausten et al., Reference Hausten, Gillis and Pernicka2010), although large-scale mining did not become established until Roman times (Mehang et al., Reference Mehang, Edwards, Schofield, Raab, Feldmann, Moran, Bryant, Thornton and Dawson2012). Effluent from these mines and the leachates from the mine spoil heaps entered rivers and estuaries and affected marine life (Garnacho et al., Reference Garnacho, Tyler and Peck2001; Daka & Hawkins, Reference Daka and Hawkins2004). Seasonal variation in the copper content of coastal seawater was measured (Atkins, Reference Atkins1953) and probably reflected periods of increased river flow. Drainage waters from coal mines were also shown to have adverse effects on estuarine fauna (Woolsey & Wilkinson, Reference Woolsey and Wilkinson2007).
Several papers describe the adaptation of invertebrates to living in sediments containing high concentrations of heavy metals, including lead (Daka & Hawkins, Reference Daka and Hawkins2004), manganese (Bryan & Hummerstone, Reference Bryan and Hummerstone1973a), mercury (Clark & Topping, Reference Clark and Topping1989) and zinc (Daka & Hawkins, Reference Daka and Hawkins2004). Common estuarine organisms were studied as indicator species to be analysed for metal contamination in estuaries. These included the brown seaweed Fucus vesiculosus (Bryan & Hummerstone, Reference Bryan and Hummerstone1973b), the barnacle Balanus improvisus (Rainbow et al., Reference Rainbow, Smith and Lau2002), the bivalve Scrobicularia plana (Bryan & Hummerstone, Reference Bryan and Hummerstone1978) and the gastropod Littorina littorea (Bryan et al., Reference Bryan, Langston, Hummerstone, Burt and Ho1983; Mason & Simkiss, Reference Mason and Simkiss1983).
Papers have described the physiological and behavioural effects of copper (Blaxter, Reference Blaxter1977; Manley, Reference Manley1983; Garnacho et al., Reference Garnacho, Tyler and Peck2001) and its effects on larval development (Ruiz et al., Reference Ruiz, Bryan, Wigham and Gibbs1996). Concentrations of copper and zinc, just above the respective maxima of 25 and 138 nM, found in seawater from the English Channel, were shown to inhibit carbon fixation rates in phytoplankton (Davies & Sleep, Reference Davies and Sleep1979, Reference Davies and Sleep1980) and low concentrations of mercury (5 µM) inhibited algal cell division (Davies, Reference Davies1976).
Some of the above studies led to separate MBA publications on biological indicator species for heavy metal contamination in estuaries (Bryan et al., Reference Bryan, Langston and Hummerstone1980) and on the history of heavy metal pollution of the Fal estuary due to mining activity (Bryan & Gibbs, Reference Bryan and Gibbs1980). The papers published in the JMBA made an important contribution to a review of the effect of trace metals on organisms in the British Isles (Rainbow, Reference Rainbow2018).
Tributyl tin (TBT)
Following the introduction of marine anti-fouling paints containing tributyl tin (TBT) compounds, populations of the dogwhelk, Nucella lapillus, showed marked declines in numbers in the vicinity of harbours and marinas (Bryan et al., Reference Bryan, Gibbs and Hummerstone1986, Reference Rees, Waldock, Mathiessen and Pendle1987, Reference Bryan, Bright, Hummerstone and Burt1993). The cause of the decline was found to be the development of a penis and vas deferens on the female, in response to TBT exposure. The vas deferens overgrowing the vulva prevented the release of egg capsules and thus rendered the female sterile (Bryan et al., Reference Bryan, Gibbs and Hummerstone1986, Reference Bryan, Gibbs, Burt and Hummerstone1987). Other gastropods were similarly affected (Gibbs et al., Reference Gibbs, Bryan, Pascoe and Burt1991; Kohn & Almasi, Reference Kohn and Almasi1993; Averbuj & Penchaszadeh, Reference Averbuj and Penchaszadeh2010). The accumulating evidence of the damage caused by these antifouling paints led to the UK banning the use of the antifouling agent on vessels <25 m length overall in 1986, although larger vessels could still use it. An EU ban on the presence of TBT-based antifouling coatings on ship hulls in EU ports was introduced in January 2008 and a global ban on both the application and presence on ship hulls of TBT-based antifoulants the following September. The recovery of gastropod populations in affected areas in Watermouth Cove, an area affected by leaching from the hulls of pleasure craft, was described (Crothers, Reference Crothers2003): even outside the cove, it took 13 years for the population of Nucella lapillus to recover. In areas where there were no nearby unaffected populations and scarce natural habitat, artificial hard substrata provided stepping-stones that facilitated the migration of Nucella (Bray et al., Reference Bray, McVean, Nelson, Herbert, Hawkins and Hudson2012).
The early reports regarding the effects of TBT pollution formed the basis for longer-term recovery studies. At a site near a shipyard in Falmouth, Cornwall, the population of the gastropod Ocenebra erinacea was still severely affected 20 years after initial exposure (Gibbs, Reference Gibbs2009). The TBT concentration in sediments off Southampton had only halved after 33 years (Langston et al., Reference Langston, Pope, Davey, Langston, O'Hara, Gibbs and Pascoe2015). Reviews in other recent papers on stress in intertidal communities and the global impacts of TBT also cite early studies published in the JMBA (Crowe et al., Reference Crowe, Thompson, Bray and Hawkins2000; Sousa et al., Reference Sousa, Takahashi and Pastorhino2014).
Discussion
Papers published in the JMBA, since 1887, reveal the development of collection, measurement and survey techniques, as well as our understanding of the marine ecosystem and the inter-relationships of different organisms from viruses to cetaceans. These papers are now cited in modern texts on marine ecology (Barnes & Hughes, Reference Barnes and Hughes1999; Kaiser et al., Reference Kaiser, Attrill, Jennings, Thomas, Barnes, Brierley, Hiddink, Kaartokallio, Polunin and Raffaelli2011) and fisheries ecology (Jennings et al., Reference Jennings, Kaiser and Reynolds2001) as well as in manuals on sampling methods in the marine environment, e.g. Eleftheriou (Reference Eleftheriou2013).
The long series of records of changes in the distributions and densities of species, together with corresponding data on the physical and chemical environment, has allowed the development of models to forecast future ecosystem changes in different areas of the oceans (Zacharias et al., Reference Zacharias, Morris and Howes1999; Huang & Brooke, Reference Huang and Brooke2011; Hattab et al., Reference Hattab, Lasram, Albouy, Sammari, Romdhane, Cury, Leprieur and Le Loçh2013).
Since the earliest records, marine organisms have been affected by both cyclical climatic changes and anthropogenic discharges and pollutants. Disentangling the causes of ecosystem changes resulting from multiple stressors is complicated. A recent analysis (Hawkins et al., Reference Hawkins, Evans, Mieszkowska, Adams, Bray, Burrows, Firth, Genner, Leung, Moore, Pack, Schuster, Sim, Whittington and Southward2017a) stresses the need for continued long-term observations and reveals the value of the early ecosystem studies published in the JMBA. The records published in the JMBA have been especially useful in describing the changes in the English Channel ecosystem through periods of warming and cooling, as well as describing the effects of and the recovery from pollution events (Southward et al., Reference Southward, Langmead, Hardman-Mountford, Aiken, Boalch, Dando, Genner, Joint, Kendall, Halliday, Harris, Leaper, Mieszkowska, Pingree, Richardson, Sims, Smith, Walne and Hawkins2005).
The JMBA started as a ‘house journal’ for the members of the Marine Biological Association of the United Kingdom, publishing work carried out at the Plymouth Laboratory by members, staff and visiting scientists. It has evolved, by the 100th volume, to become a truly international journal for marine science, publishing papers from around the world dealing with the marine environment and marine biota, from the intertidal to the hadal zone. The JMBA continues to contribute to our knowledge on how changing climates may affect our oceans in future. One uncertainty, at the time of writing, is how the proposals, by major funding agencies, that all future scientific publications resulting from their grants must be in fully open access journals (McNutt, Reference McNutt2019), will affect the JMBA.
Acknowledgements
We thank Barbara Bultman, the National Marine Biological Library and Jane Lewis for assistance during the writing of this review. Steve Hawkins gave us helpful comments on an earlier version of the manuscript. We thank the reviewers for their helpful comments.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.












