Article contents
Theoretical study on Sn–Sb-based lead-free solder by ab initio molecular dynamics simulation
Published online by Cambridge University Press: 07 May 2019
Abstract
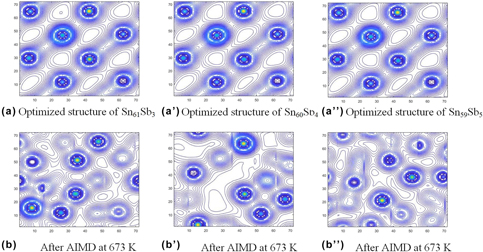
Sn–Sb alloy is an ideal candidate for lead-free solder; however, its performance has been inferior to that of Sn–Pb alloy. Here, the authors used ab initio molecular dynamics simulation to investigate the interatomic interaction in Sn–Sb-based lead-free solders. By calculating the electron density distribution, bond population, and partial density of states, the authors found that the Sn–Sb bonds are a mixture of nonlocalized metal and localized covalent bonds. The covalent bond between Sn and Sb is easy to break at higher temperatures, so Sn–Sb (6.4 wt%) had better fluidity than other studied Sn–Sb alloys. Furthermore, adding Cu or Ag into Sn–Sb alloys can decrease the strength of covalent bonds and stabilize the metal bonds, which improves the metallicity and wettability of the Sn–Sb–Cu and Sn–Sb–Cu–Ag systems when the temperature increases. These results are all in good agreement with experimental findings and have significant value for the development of new solder alloys.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © Materials Research Society 2019
References
- 6
- Cited by




