Article contents
Synthesis of highly dispersed Pd nanoparticles with high activity for formic acid electro-oxidation
Published online by Cambridge University Press: 30 May 2013
Abstract
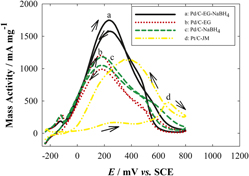
To obtain highly dispersed and active Pd/C catalysts for formic acid electro-oxidation (FAEO), carbon supported Pd nanoparticles were prepared by three different synthesis methods. The first Pd deposition technique went through the ethylene glycol (EG)-assisted sodium borohydride (NaBH4) reduction process (Pd/C-EG-NaBH4). The second method was the polyol process (Pd/C-EG). The third method was the general NaBH4 reduction process (Pd/C-NaBH4). The results of x-ray diffraction and transmission electron microscopy (TEM) show that the Pd particles on carbon black prepared by the first method have the smallest average size of 3.5 nm with a narrow size distribution. Cyclic voltammetry was used to characterize the electrochemical performance. The peak current density (mass activity) of the Pd/C-EG-NaBH4 for FAEO is 1742 mA/mgPd, which is 1.45 times higher than that of Pd/C-EG and 1.48 times higher than that of Pd/C-NaBH4. Moreover, the experimental results show that the first method has no dependence on the pH value of the synthesis procedure, while the other two methods in the literature are greatly affected by the pH value.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2013
References
REFERENCES
- 7
- Cited by




