Article contents
On the steady-state chemical potential profiles in bilayer solid electrolytes
Published online by Cambridge University Press: 05 July 2012
Abstract
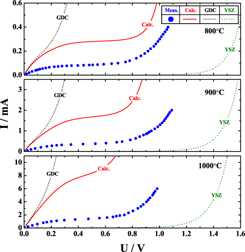
All the existing theories presuppose the continuity of oxygen chemical potential at interfaces between contiguous layers, in calculating steady-state chemical potential profiles across a multilayer composite of mixed conductor oxides that is subjected to an oxygen chemical potential gradient of whatsoever origin, but they have never been tested experimentally. We have observed that this continuity hypothesis appears to break down in yttria-stabilized zirconia/gadolinia-doped ceria bilayer electrolytes under an electric tension in ion-blocking condition (Hebb–Wagner polarization). It is suggested that all the continuity hypothesis-based existing theories to calculate the steady-state chemical potential distributions may not always be true depending on boundary conditions.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 27 , Issue 15: Focus Issue: Advanced Materials for Fuel Cells , 14 August 2012 , pp. 1969 - 1974
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 2
- Cited by




