Article contents
Facile synthesis of Pt–Ag octahedral and tetrahedral nanocrystals with enhanced activity and durability toward methanol oxidation
Published online by Cambridge University Press: 08 October 2018
Abstract
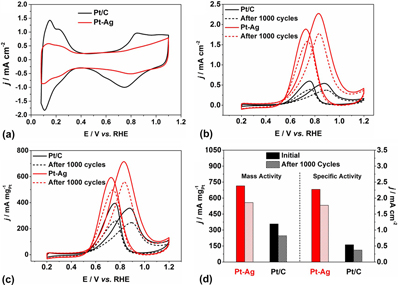
Engineering the surface structure, together with the incorporation of a second metal, is an effective strategy for boosting the catalytic activities of Pt-based catalysts toward various reactions. Here, we report a facile approach to the synthesis of Pt–Ag octahedral and tetrahedral nanocrystals covered by concave surfaces. The presence of the Ag(I) precursor not only facilitated the reduction of the Pt(IV) precursor but also led to the formation of concaved facets on the Pt–Ag nanocrystals. Besides, poly(vinylpyrrolidone) (PVP) was demonstrated to serve as a co-reductant, in addition to its role as a colloidal stabilizer. Using PVP with different molecular weights, we were able to tune the size of the Pt–Ag nanocrystals in the range of 9–25 and 14–32 nm for the octahedral and tetrahedral shapes, respectively. The Pt–Ag nanocrystals exhibited 4.6- and 2.0-fold enhancements in terms of specific and mass activities, respectively, toward methanol oxidation, when benchmarked against the commercial Pt/C catalyst. After 1000 cycles of the accelerated tests, the specific and mass activities of the Pt–Ag nanocrystals were still 3.6 and 1.6 times as high as those of the original commercial Pt/C.
Keywords
- Type
- Invited Feature Paper
- Information
- Copyright
- Copyright © Materials Research Society 2018
Footnotes
This paper has been selected as an Invited Feature Paper.
References
REFERENCES
- 3
- Cited by




