Article contents
Energy conversion systems: Molecular architecture engineering of metal precursors and their applications to vapor phase and solution routes
Published online by Cambridge University Press: 21 September 2020
Abstract
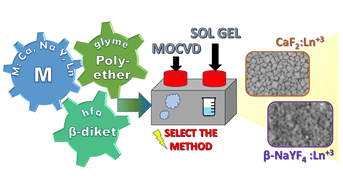
A careful engineering of the central metal coordination spheres provides adducts with excellent properties for application as precursors in vapor phase and solution processes. The family of precursors under study concerns the fluorinated metal-organic β-diketonates of alkaline, alkaline-earth and rare-earth metals adducted with a polyether, with general formula M(hfa)n·L (M = Ca, Na, Y, Yb, Er, Tm; Hhfa = 1,1,1,5,5,5 hexafluoroacetylacetone, L = diglyme or tetraglyme). Mass transport properties, such as volatility and thermal stability, of these adducts have been deeply analyzed through thermogravimetric analysis and differential scanning calorimetric measurements. These properties are rationalized in relation to the metal coordination sphere in the precursors and their applications. In particular, the precursors under focus have been applied to metal organic chemical vapor deposition and a combined sol–gel/spin-coating approach. Both methods allow us to obtain selectively and reproducibly CaF2 and NaYF4 phases with several combinations of lanthanide doping ions, using a proper mixture of fluorinated precursors. A careful optimization of both synthetic strategies is the key point for the production of different lanthanide-doped binary and multicomponent fluoride films, i.e., CaF2:Yb3+,Er3+; CaF2:Yb3+,Tm3+; CaF2:Yb3+,Er3+,Tm3+ and NaYF4:Yb3+,Er3+; NaYF4:Yb3+,Tm3+, with suitable morphologies, compositions and crystalline structures. The films show very promising upconversion properties, thus pointing to their appealing applications in photovoltaic systems and white light emission devices.
- Type
- Invited Feature Paper
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 10
- Cited by





