Article contents
Effect of transition metal ion doping on photocatalytic properties of In–Ti oxides
Published online by Cambridge University Press: 10 November 2015
Abstract
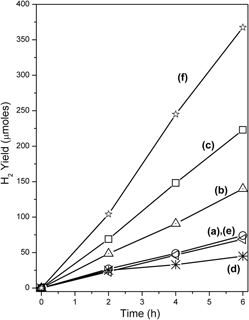
Nominal compositions of In2Ti1−xTmxO5−δ, (0.0 ≤ x ≤ 0.2 and Tm = Fe3+ and Cr3+) were synthesized by ceramic route and characterized by relevant techniques. In2Ti1−xFexO5−δ samples were single phased isomorphic with In2TiO5 phase while Cr substitution has resulted in mixed phased compositions. Dynamic light scattering experiments showed the increase in proportion of smaller sized particles with the increase in extent of Fe substitution. Mössbauer spectra revealed that Fe exists in +3 oxidation state in substituted oxides. The band gap decreased from 3.2 (In2TiO5) to 2.1 eV (In2Ti0.8Fe0.2Ox−δ) and to 1.9 eV in case of Cr substitution (In2Ti0.8Cr0.2O5−δ) attributed to participation of Fe 3d/Cr 3d levels. Photocatalytic H2 generation was evaluated over the substituted oxides from water–methanol mixtures under UV–visible irradiation. The decreasing order of photocatalytic activity is as follows: 20% Fe (mol%) > 10% Fe > indium titanate ≈ 20% Cr > 10% Cr. The loading of Pt as cocatalyst on surface of most active photocatalyst, In2Ti0.8Fe0.2Ox−δ further enhanced the photocatlytic H2 yield.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
Footnotes
Contributing Editor: Xiaobo Chen
References
REFERENCES
- 2
- Cited by




