Article contents
Different crystallization mechanisms in polypropylene–nanoclay nanocomposite with different weight percentage of nanoclay additives
Published online by Cambridge University Press: 20 March 2012
Abstract
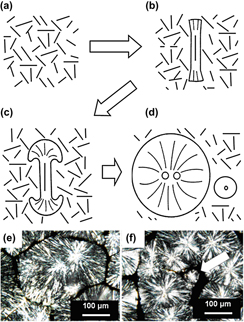
Polypropylene–clay (Cloisite Na+) composites with clay contents in weight percentage (wt%) ranging from 1 to 15% were characterized for crystallization mechanism and kinetics. Combination of differential scanning calorimetry, transmission electron microscopy (TEM), and polarized light microscopy was used to investigate the crystallization behavior. Different crystallization mechanisms were observed in the matrix with 1–5 wt% nanoclay compared to the matrix with 10 and 15 wt% of nanoclay additive. TEM micrographs revealed intercalated and flocculated morphology for all the concentrates. At lower wt%, well-dispersed clay platelets acted as antinucleating agent and reduced polymer chain mobility. At high wt%, nucleation rate overcomes the slow diffusion rate. In the case of samples with higher wt% of nanoclay additives, segregation and precipitation of clay was observed in the interspherulite region. On the basis of crystallization kinetics and morphology results, a schematic model of the nanocomposite formation is proposed.
Keywords
- Type
- Articles
- Information
- Journal of Materials Research , Volume 27 , Issue 10: Focus Issue: Crystallization Processes in Polymer-Based Materials , 28 May 2012 , pp. 1360 - 1371
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 10
- Cited by




