Article contents
Characterization of a new Ca–Cd hydroxide hydrothermally synthesized and its implications for cement isolation of Cd
Published online by Cambridge University Press: 31 January 2011
Extract
Mixtures of CaO–CdO (1 : 1) were hydrothermally treated in a pressure reactor at 200 °C and 200 psi of pressure during a period of 16 h. The evolution of the reaction was followed by x-ray diffraction (XRD), infrared spectroscopy (IR), and thermogravimetric (TG and DTG) analysis. Also, the composition of the filtered solutions was analyzed to determine the mechanism of the reaction as well as the thermodynamic solubility constant of the new compound formed. The results show that CaO and CdO react, giving rise to a new CaCd(OH)4 hydroxide whose thermodynamic solubility constant, 1.5 ± 0.4 × 10−11 M2, is six orders of magnitude lower than those of both Ca(OH) 2 and β–Cd(OH) 2. This low solubility constant justifies the Cd2+ concentration measured in the pore solution of cement matrices used to immobilize cadmium containing wastes. The mechanism of the reaction proposed is via dissolution of both Ca(OH) 2 and β–Cd(OH)2, Ca2+ and 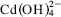 being the predominant species in solution.
being the predominant species in solution.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 1998
References
- 4
- Cited by




