Article contents
Autophagy inhibitior autophagy-related 7 small interfering RNA and doxorubicin dual-loaded nanostructured lipid carrier to combat multidrug resistance
Published online by Cambridge University Press: 25 November 2020
Abstract
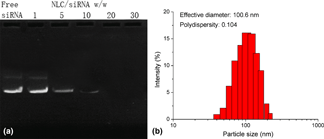
The multidrug resistance (MDR) is a widely observed phenotype that contributed to the major obstacle of impairing the outcome of cancer chemotherapy. With the aim to reverse MDR in the breast cancer cell line, the autophagy-related 7 (ATG7) small interfering RNA (siRNA) capable of downregulating the cellular autophagy level was loaded into a cationic nanostructured lipid carrier (NLC) with doxorubicin (Dox) to build a platform (NLC/D-R) for effective chemotherapy of breast cancer. Our results revealed that NLC/D-R was well-dispersed nanoparticles with satisfy protection to siRNA. In addition, NLC/D-R also exerted a sufficient drug release of both cargos under an acidic environment with high stability and biocompatibility at the physiological environment. Furthermore, NLC/D-R showed a preferable transfection profile to PEI 25k. The downregulated autophagy level in NLCF-7/Adr cells resulted in reverse of MDR and accumulated Dox retention in cells. The in vitro cytotoxicity using both cells on flat surfaces and multicellular tumor spheroid (NLCTS) model confirmed that NLC/D-R showed much elevated anticancer performance than NLC/Dox or NLC/siRNA, which suggested the synergistic effect between anti-autophagy and chemotherapy.
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 3
- Cited by





