Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Koh, S. K.
Choi, S. C.
Han., S.
and
Jung, H-J
1997.
Ion Assisted Reaction In Polymer And Ceramics.
MRS Proceedings,
Vol. 504,
Issue. ,
Smith, L. L.
Arthur, C. B.
Yang, C. S.
and
Parsons, G. N.
1998.
Substrate Surface Morphology and Growth Evolution of Low Temperature Silicon Nitride on Transparent Plastics.
MRS Proceedings,
Vol. 508,
Issue. ,
Sarto, F.
Alvisi, M.
Melissano, E.
Rizzo, A.
Scaglione, S.
and
Vasanelli, L.
1999.
Adhesion enhancement of optical coatings on plastic substrate via ion treatment.
Thin Solid Films,
Vol. 346,
Issue. 1-2,
p.
196.
Choi, Sung-Chang
Choi, Won-Kook
Jung, Hung-Jin
Park, Jung-Gyu
Chung, Bong-Chul
Yoo, Young-Sook
and
Koh, Seok-Keun
1999.
Relation between hydrophilicity and cell culturing on polystyrene Petri dish modified by ion-assisted reaction.
Journal of Applied Polymer Science,
Vol. 73,
Issue. 1,
p.
41.
Choi, W.K
Choi, S.C
Jung, H.-J
and
Koh, S.K
1999.
Ceramic surface modification by a keV ion irradiation.
Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms,
Vol. 148,
Issue. 1-4,
p.
740.
Choi, S.C.
Han, S.
Choi, W.K.
Jung, H.-J.
and
Koh, S.K.
1999.
Hydrophilic group formation on hydrocarbon polypropylene and polystyrene by ion-assisted reaction in an O2 environment.
Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms,
Vol. 152,
Issue. 2-3,
p.
291.
Rhee, Kyong Y.
Choi, Nak‐Sam
and
Park, Soo‐Jin
2002.
Surface treatment of CFRP composites by Ar+ irradiation to improve bonding strength between aluminum and CFRP composites.
Polymer Composites,
Vol. 23,
Issue. 6,
p.
1151.
Wada, M.
Nishigaito, S.
Flauta, R.
and
Kasuya, T.
2003.
Modification of bamboo surface by irradiation of ion beams.
Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms,
Vol. 206,
Issue. ,
p.
557.
Rhee, K.Y.
Choi, J.H.
and
Park, S.J.
2005.
Effect of 1keV Ar+ irradiation on the residual strength of PE fiber-reinforced composites.
Materials Science and Engineering: A,
Vol. 395,
Issue. 1-2,
p.
288.
Han, Younggun
Kim, Donghwan
Cho, Jun-Sik
Beag, Young-Whan
Koh, Seok-Keun
and
Chernysh, Vladimir S.
2005.
Effects of substrate treatment on the initial growth mode of indium-tin-oxide films.
Journal of Applied Physics,
Vol. 97,
Issue. 2,
Han, Younggun
Kim, Donghwan
Cho, Jun-Sik
Beag, Young-Whan
and
Koh, Seuk-Keun
2006.
Study of the substrate treatment effect on initial growth of indium tin-oxide films on polymer substrate using in situ conductance measurement.
Thin Solid Films,
Vol. 496,
Issue. 1,
p.
58.
Kim, Dong-Ho
Park, Mi-Rang
Lee, Hak-Jun
and
Lee, Gun-Hwan
2006.
Thickness dependence of electrical properties of ITO film deposited on a plastic substrate by RF magnetron sputtering.
Applied Surface Science,
Vol. 253,
Issue. 2,
p.
409.
Singh, N.L.
Qureshi, Anjum
Mukherjee, S.
Rakshit, A.K.
Tripathi, A.
and
Avasthi, D.K.
2007.
Surface modification of polymeric blends by nitrogen plasma implantation.
Surface and Coatings Technology,
Vol. 201,
Issue. 19-20,
p.
8278.
Panwar, Amrish K
Barthwal, S K
Shivaprasad, S M
and
Ray, S
2008.
Physico-chemical characterization of a polycarbonate (PC) surface modified by exposure to dc glow discharge in air.
Journal of Physics D: Applied Physics,
Vol. 41,
Issue. 13,
p.
135305.
Song, Jeom Sik
Lee, Sukmin
Jung, Seong Hee
Cha, Gook Chan
and
Mun, Mu Seong
2009.
Improved biocompatibility of parylene‐C films prepared by chemical vapor deposition and the subsequent plasma treatment.
Journal of Applied Polymer Science,
Vol. 112,
Issue. 6,
p.
3677.
Qureshi, Anjum
Shah, Sejal
Pelagade, S
Singh, N L
Mukherjee, S
Tripathi, A
Despande, U P
and
Shripathi, T
2010.
Surface modification of polycarbonate by plasma treatment.
Journal of Physics: Conference Series,
Vol. 208,
Issue. ,
p.
012108.
Pelagade, S
Singh, N L
Shah, Sejal
Qureshi, Anjum
Rane, R S
Mukherjee, S
Deshpande, U P
Ganesan, V
and
Shripathi, T
2010.
Surface free energy analysis for bipolar pulsed argon plasma treated polymer films.
Journal of Physics: Conference Series,
Vol. 208,
Issue. ,
p.
012107.
Yaghoubi, Houman
and
Taghavinia, Nima
2011.
Surface chemistry of atmospheric plasma modified polycarbonate substrates.
Applied Surface Science,
Vol. 257,
Issue. 23,
p.
9836.
Zhao, Wenxia
and
Wang, Zenglin
2013.
Adhesion improvement of electroless copper to PC substrate by a low environmental pollution MnO2–H3PO4–H2SO4–H2O system.
International Journal of Adhesion and Adhesives,
Vol. 41,
Issue. ,
p.
50.
Ovtsyn, A A
Smirnov, S A
Shikova, T G
and
Kholodkov, I V
2017.
Modification of polycarbonate surface in oxidizing plasma.
Journal of Physics: Conference Series,
Vol. 927,
Issue. ,
p.
012038.
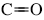 bond (carbonyl group). In atomic force microscopy (AFM) study, the root mean square of surface roughness was changed from 14 Å to 22–27 Å by Ar+ ion irradiation without flowing oxygen gas and 26–30 Å by Ar+ ion irradiation with flowing 4 sccm oxygen gas. It was found that wettability of the modified PC surface was not greatly dependent on the surface morphology, but on an amount of hydrophilic group formed on the surface in the ion beam process.
bond (carbonyl group). In atomic force microscopy (AFM) study, the root mean square of surface roughness was changed from 14 Å to 22–27 Å by Ar+ ion irradiation without flowing oxygen gas and 26–30 Å by Ar+ ion irradiation with flowing 4 sccm oxygen gas. It was found that wettability of the modified PC surface was not greatly dependent on the surface morphology, but on an amount of hydrophilic group formed on the surface in the ion beam process.



