Article contents
Ab-initio molecular characterization of nonclassical fullerenes cluster using two probe approach
Published online by Cambridge University Press: 05 January 2017
Abstract
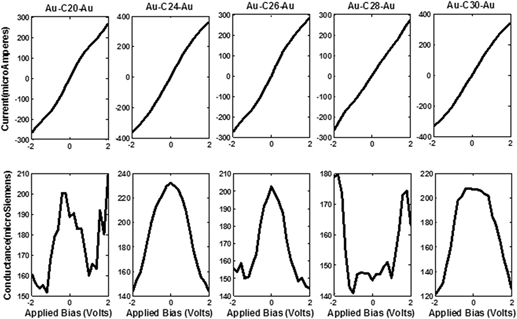
We outline the scrutiny of the two probe devices formed by placing the nonclassical fullerene molecules CM (20 ≤ M ≤ 30) within semi-infinite gold electrodes using density functional theory. The electronic structure and molecular orbitals of isolated fullerene molecules are broadened to form junction devices with charge injection at zero as well as variegated bias respectively. The molecular junctions thus formed are contemplated for two important electrical constitutions, current, and conductance. These parameters are then elaborated and contemplated for their electronic parameters namely, the density of states, transmission coefficient, molecular orbitals, molecular projected self-consistent Hamiltonian states, electron density, and Mulliken population. We conclude that C20 and C24 fullerene molecule exhibits extremely metallic behavior while others fail to demonstrate such behavior. The molecular device, thus formed, strongly supports the superconductive behavior of the C24 molecule with an ability to easily adapt by modulating its active molecular orbitals under applied potential.
Keywords
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2017
References
REFERENCES
- 6
- Cited by





