Introduction
Taenia solium infection in humans as definitive hosts of the parasite leads to taeniasis and excretion of parasite eggs in faeces. Following the ingestion of tapeworm eggs, the intermediate hosts (pigs, as well as humans) develop cysticercosis. Taenia solium infection is a significant medical problem in many countries of Central and South America, Africa and Asia (Coral-Almeida et al., Reference Coral-Almeida, Gabriël, Abatih, Praet, Benitez and Dorny2015), although cases (autochthonous or imported) have been registered in almost all countries of Europe (Zammarchi et al., Reference Tumolskaia, Glazunova, Dobzhanskiĭ, Sushurov and Stankov2013; Devleesschauwer et al., Reference Devleesschauwer, Allepuz and Dermauw2017) and North America (Serpa & White, Reference Rodriguez-Canul, Fraser, Allan, Dominguez-Alpizar, Argaez-Rodriguez and Craig2012). The territory of the former Union of Soviet Socialist Republics (USSR), which comprises the largest part of the Russian Federation (RF) today, was traditionally considered an endemic area for T. solium infection. The newest data consider the RF subendemic for this infection (World Health Organization, Reference Trevisan, Sotiraki and Laranjo-González2015).
Almost 100 years ago, infection with T. solium (and Taenia saginata) tapeworms was identified as an important health and economic problem in USSR (Chernikova et al., Reference Chernikova, Migliorini, Litvinov, Darchenkova and Novozhilov2015). A programme for the prevention of taeniasis was established in 1960, when a methodological guide for the differential morphologically based diagnosis of T. saginata and T. solium taeniasis during microscopic examination of faeces was also issued. The programme included active detection and prevention of the spread of taeniasis through routine preventive examinations of individuals professionally engaged in the production, storage, transport and sale of food and drinking water, as well as in childcare and education. Preventive measures include the reporting and epidemiological investigation of cases to discover infection sources, as well as wastewater treatment and the development of diagnostic capacities and therapies. Treatment is carried out under physicians’ control, with mandatory coprological examination upon completion of the therapy course. As part of the prevention programme, mandatory inspection of meat in slaughterhouses, shops (markets, food shops) and meat processing plants is the task of the veterinary service (Rosselkhoznadzor, 1988). Inspection includes palpation of the tongue, as well as visual inspection of the incised external and internal masticatory and cardiac muscles. If the result is positive, the lumbar, cervical, scapular-ulnar (anconaeus), dorsal and pelvic muscles as well as the diaphragm are also cut and examined. When more than three live or dead cysticerci are discovered in a 40 cm2 area of the incision into a predilection muscle or in at least one muscle of the carcass, the carcass is destroyed. If up to three cysticerci are found in the predilection muscles, and up to three on the incisions of the other muscles of the trunk, the head and internal organs are destroyed, and the trunk is disinfected. In both cases, the fat is disinfected by heating, freezing or salting (Rosselkhoznadzor, 1988). The meat is disinfected by freezing, salting or heating. Freezing is prescribed to last for ten days in chambers at −12°C to achieve a temperature of −10°C in the muscles, at a depth of 7–10 cm, or four days in chambers at −13°C and −12°C in muscles. For salting, pieces of meat weighing no more than 2.5 kg are covered with table salt to the amount of 10% meat weight, and then placed in a solution with a concentration of at least 24% table salt and stored for 20 days. Meat which has been subjected to salting and freezing must not be sold and used in households, but, rather, exclusively processed into meat products in industrial plants whose production includes heat treatment at temperatures of 80 to 120°C for 90 to 150 min, so that the temperature inside the meat portion reaches 75 to 80°C. Although previously inspected and branded at the slaughter point, carcasses (halved or quartered) are delivered for sale to markets, where they are also subject to veterinary inspection (Rosselkhoznadzor, 1988). In case of detection of infected meat, or when the source of infection is established after the obligatory reporting of a taeniasis case, if possible, the farm is inspected, including the examination of staff and livestock, with additional disinfection measures implemented.
The implementation of preventive measures, which continued over the following decades until the collapse of the USSR in 1991, had significantly reduced the incidence of taeniasis (Guzeeva, Reference Gorokhov, Skira and Klenova2011). The implementation of the same prevention programme was continued after 1991 in the RF, but political changes and, more importantly, negative trends in economic life during the 1990s undoubtedly led to dysfunction in all sections of society, including areas of health and veterinary prevention. This has resulted in an increase in the frequency of T. solium taeniasis in several areas of the RF, especially in the European part of the country (Kiselev et al., Reference Khudyakova2014), leading to a 0.2% incidence of taeniasis during the 1990s (Onishchenko, Reference Kosminkov2007). After 2000, the beginning of the economic recovery (according to World Bank GDP data, https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=RU) of the RF provided a prerequisite for more stringent implementation of the prevention programme, but no data on the epidemiological characteristics of T. solium infection in these new circumstances exist, even in the latest reviews (Trevisan et al., Reference Serpa and White2018).
The goal of this study was to collect and analyse epidemiological data on T. solium infection through a systematic review of scientific and grey literature, including official reports in RF for the 2000–2019 period.
Methods
Search strategy
In compliance with PRISMA guidelines (Moher et al., Reference Klimenko, Tretyakova, Vasilenko, Svinaryov and Svyatogorov2009), we conducted a systematic database review of published literature and locally published sources of information on epidemiology (occurrence, prevalence, incidence, age, gender and geographical distribution) of T. solium infection (taeniasis and cysticercosis) in the human and animal populations in the RF, published between 2000 and 2020 (supplementary file 1: table S1; file 2: text S1).
Databases and other sources
Both international and Russian databases were searched for published data and grey literature (including master and doctoral theses). For published data, we searched three international databases (PubMed – http://www.ncbi.nlm.nih.gov/pubmed; Web of Science – http://ipscience.thomsonreuters.com/product/web-of-science; and Google Scholar – https://scholar.google.com/scholar), and three Russian databases (DVGMU Library, Far Eastern Library of the Medical State University – www.fesmu.ru/elib/Search.aspx?Catalog=1; eLIBRARI.RU, Scientific Electronic Library – https://elibrari.ru/; and Ciberleninka – https://ciberleninka.ru/). In addition, the international OpenGrey (http://www.opengrey.eu/) and Russian Scientific Library, Library of dissertations (http://freereferats.ru/index.php?cat=91&page=14) databases were searched. The following search phrases were used: neurocysticercosis OR cysticercosis OR pork tapeworm OR taenia OR solium OR taeniasis AND Russia. For Russian databases, the same search phrases were used only in Russian. In addition, we searched the official websites of the Russian government services for official reports and reviews, as well as epidemiological bulletins, using cysticercosis, neurocysticercosis (NC) or taeniasis (in Russian) as search phrases. Data on GDP per capita/per year in the RF were extracted from the World Bank reports, as well as data on the population, GDP per capita per districts, population or pig breeding/slaughter from reports by the Russian Federal State Statistics Service – Rosstat (all available online).
Selection criteria
Search results were compiled and rechecked for publication date (2000–2020) as well as duplicates, with inadequate records being removed. Titles and abstracts were then screened for relevance, applying the following exclusion criteria: (1) studies concerning a parasite other than T. solum; (2) studies conducted outside of the RF; (3) studies not containing data for the study period; (4) studies presenting data not associated with the epidemiological characteristics of T. solium infection but rather focusing on clinical features, therapy or parasite biology; (5) studies including a general review of the topic, without original data; and (6) studies presenting duplicate data. The same exclusion criteria were applied to full text; an additional exclusion criterion for these was if they only repeated the epidemiological data published in official reports. Official reports were considered acceptable data sources.
Data extraction and generation
Prevalence data reported in the selected literature and reports were used directly. The prevalence was calculated in cases where the number of cases of taeniasis was reported, using the official census data for population size, and, in the case of cysticercosis of pigs, the number of slaughtered pigs in that year according to official reports was used as the denominator. Statistical analysis was carried out using univariate analysis of variance, Pearson's two-tailed correlation and the chi-square (χ2) test, as appropriate.
Results
Search results
The search strategy is outlined in the PRISMA 2009 flow diagram (supplementary file 3: fig. S1). There were no masters or doctoral theses that corresponded to the search phrases. The final analysis included 12 full-text articles and 11 official reports (supplementary file 4: table S2). Data from the Russian Federal State Statistics Service and the World Bank were also used for the analysis.
Occurrence of Taenia solium infection in humans
Occurrence of taeniasis
According to official data (whether published in articles or in official reports) a total of 2889 cases of T. solium taeniasis were registered in the RF in the last 20 years (2000–2019; official data not available for 2003) (Onishchenko, Reference Kosminkov2007; Rosselkhoznadzor annual reports, Reference Rosselkhoznadzor2008–2019; Derzhavina et al., Reference Derzhavina, Boldyreva, Bukreev and Shevelyova2011; Sarbasheva et al., 2012; Dudarev et al., Reference Dudarev, Dorofeyev and Dushkina2013). The method used to diagnose taeniasis was microscopic examination of faecal samples. Of the total number of cases, 2614 were associated with the year they were registered, while for 275 cases from the Kabardino-Balkaria Republic (Sarbasheva et al., 2012), the year of detection was not specified (rather, a total was given for the 2006–2012 period), so they were not included in further analyses.
The incidence (per 100,000 population) declined by tenfold during the study period, from 0.2 in 2000 to 0.023 in 2019 (F (1,19) = 28.058, P < 0.0001) (fig. 1). Moreover, only about one-fifth of all cases were registered during the second half of the observed period (2010–2019).

Fig. 1. Number of cases of Taenia solium taeniasis per 100,000 population officially reported in the Russian Federation (2000–2019) (data for 2003 not available) (Onishchenko, Reference Kosminkov2007; Rosselkhoznadzor annual reports, Reference Rosselkhoznadzor2008–2019; Derzhavina et al., Reference Derzhavina, Boldyreva, Bukreev and Shevelyova2011; Dudarev et al., Reference Dudarev, Dorofeyev and Dushkina2013).
Some of the reported cases were imported. Thus, the only reported case in Moscow in 2010 was that of a Russian citizen infected during his stay in Turkey, and the one from 2013 was a Vietnamese citizen (Khudyakova, Reference Khudyakova2014).
The infection rate by year was negatively correlated with the GDP per capita/per year (World Bank, https://data.worldbank.org/indicator/NY.GDP.PCAP.CD?locations=RU). With the growth of GDP, the infection rate decreased and vice versa, irrespective of whether the infection rates were correlated with GDP values for the year in question (Pearson's r (N = 19) = −0.868, P = 0.000001) or for the previous year (Pearson's r (N = 19) = −0.809, P = 0.000085).
Spatial distribution of infection was not uniform. Between 2008 and 2019 (Rosselkhoznadzor annual reports, Reference Rosselkhoznadzor2008–2019), the incidence of infection was significantly higher (χ2 = 28.449, P < 0.0001) in the less populated Asian part (326 cases per ≈ 31 million population) than in the European part (390 cases per ≈ 113 million population) of RF. Analysed according to the administrative federal districts, the incidence of infection significantly varied among all eight (χ2 = 142.934, P < 0.0001) (fig. 2). Importantly, the infection rate significantly correlated with GDP per capita in the federal districts (Pearson's r = −0.826, P = 0.043) but not with the population per district (Pearson's r = −510, P = 0.301) (GDP value and the number of inhabitants for the year 2009 were used for comparison) (Rosstat, 2018, n.d.).
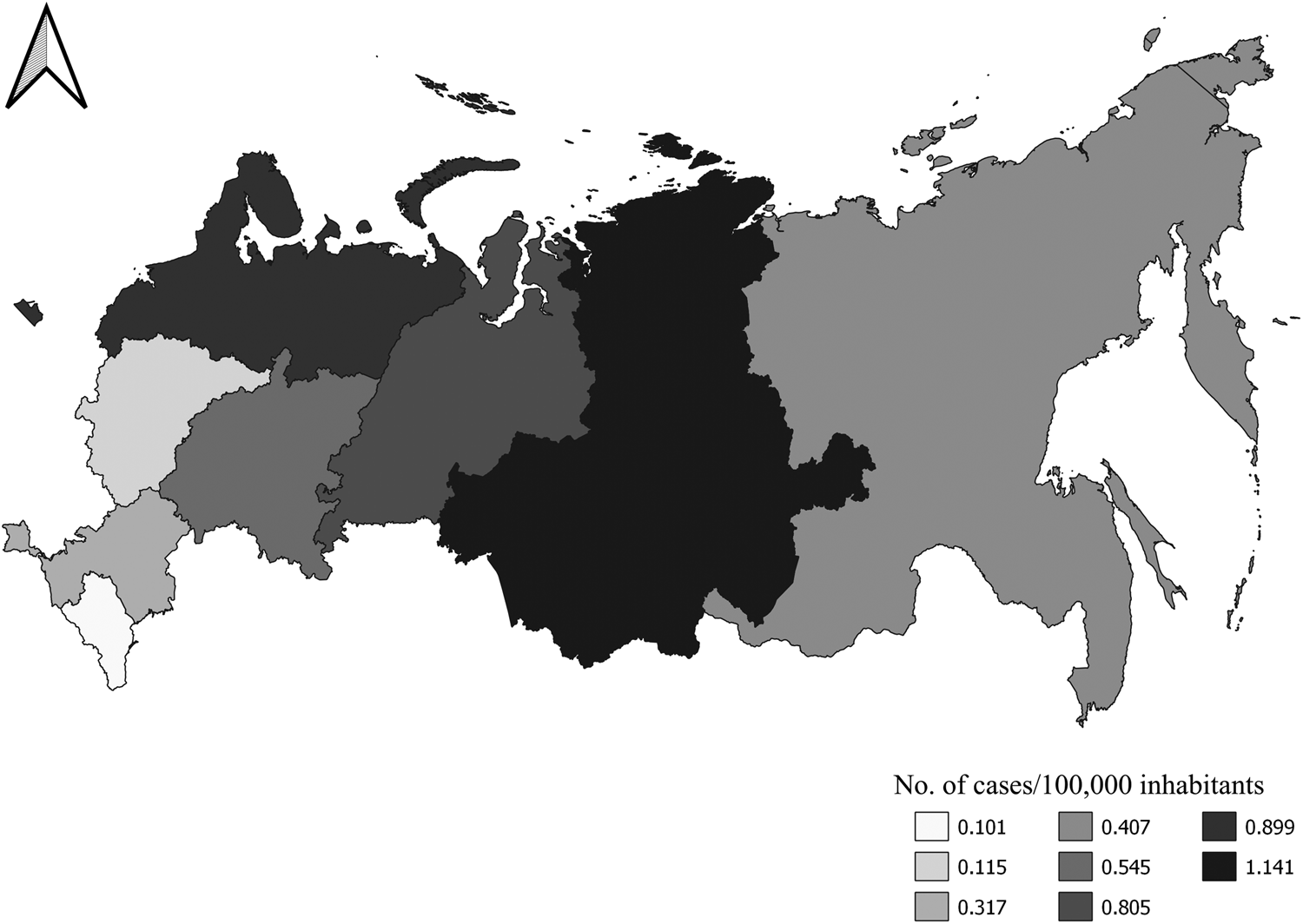
Fig. 2. Frequency of Taenia solium taeniasis by federal districts of the Russian Federation given cumulatively, for the 2008–2019 period (Rosselkhoznadzor annual reports, Reference Rosselkhoznadzor2008–2019).
The eight federal districts of the RF are divided into a total of 85 federal units. Since 2007, no cases of taeniasis have been registered in 22 of these 85 units (Rosselkhoznadzor annual reports, Reference Rosselkhoznadzor2008–2019) (fig. 3). Taeniasis was continuously registered – that is, each year, in the seven (8.2%) regions in the eastern part of the Eastern European Plain (Khakassia, Perm region, Orenburg region) or western and central Siberia (Khanty-Mansi Autonomous Area, Komi Republic, Krasnoyarsk Krai) (fig. 3). In 56, the majority of federal units, the infection was registered occasionally – that is, not every year. Specifically, less than 12 cases of infection were reported in 47 (83.9%) units, and in 38 of those, even less than five during the observed 12-year period.

Fig. 3. Reported cases of Taenia solium taeniasis by administrative units of the Russian Federation in the period 2008–2019 (Rosselkhoznadzor annual reports, Reference Rosselkhoznadzor2008–2019). Unregistered, no reported cases; occasionally registered, cases not registered every year; continuously registered, cases registered each year.
In most federal units, a decline in the incidence of infection between 2008 and 2019 was observed as an overall trend. The period was analysed in two six-year increments (2008–2013 and 2014–2019). In the federal units where the infection was continually registered, the number of cases was significantly higher during the first six-year period – that is, in the Republic of Khakassia (36/7) (χ2 = 19.56, P < 0.0001), Krasnoyarsk Krai (61/27) (χ2 = 13.16, P = 0.0003), Perm Krai (39/11) (χ2 = 15.68, P = 0.0001), Orenburg Oblast (24/9) (χ2 = 6.8181, P = 0.009) and Khanty-Mansi Autonomous Area (31/12) (χ2 = 8.3652, P = 0.0038); only in the Komi Republic (26/48) (χ2 = 6.4503, P = 0.0105) was the number of cases significantly higher during the later six-year period. For federal units where cases were registered only in certain years (e.g. in the Magadan region cases were registered in 2008, 2014 and 2015, or in Nizhny Novgorod only in 2017 and 2018) (Rosselkhoznadzor annual reports, Reference Rosselkhoznadzor2008–2019), analysis was performed cumulatively for all units; the results also showed a significantly higher number of cases during the first six-year period (252/133) (χ2 = 36.782, P < 0.0001).
The reduction of the number of federal units where the infection was registered was also evident (fig. 4) (Rosselkhoznadzor annual reports, Reference Rosselkhoznadzor2008–2019). In 2008, the infection was registered in 36, and in 2019 in 21 federal units. During the first six-year period the cumulative number of administrative units (N = 157) where the infection was registered was significantly higher than during the second six-year period (N = 117) 2014–2019 (χ2 = 4.116, P = 0.0425).

Fig. 4. Number of federal subjects of the Russian Federation in which Taenia solium taeniasis was reported (Rosselkhoznadzor annual reports, Reference Rosselkhoznadzor2008–2019).
A study of the influence of climatic and geographical characteristics on the prevalence of parasitic human infections in the Republic of Kabardino-Balkaria showed that T. solium taeniasis occurred in lower and hilly areas, but not in the mountainous areas. In contrast, in the same study, T. saginata and Trichinella spiralis infections were more prevalent in mountainous areas (Sarbasheva et al., 2012).
According to official data, the infection was mostly diagnosed (range 72–73%) in patients seeking medical care (Onishchenko, Reference Kosminkov2007, Reference Popova2010; Popova, Reference Popova2014). The remaining cases were detected during mandatory occupational health checks, such as sanitary control (24–25%), and 2–4% based on epidemiological indications (epidemiological surveillance after diagnosing cases in the surroundings). Only 1% of cases in 2009 involved individuals with a professional risk of spreading the infection (Onishchenko, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010).
The infection was more often diagnosed in women, and their share in the number of infected individuals was continuously growing (from 58% in 2009, to 69% and 66% in 2013 and 2016, respectively) (Onishchenko, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010; Popova, Reference Popova2014, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017). The greatest number of infections were registered in adults between the ages of 20 and 39 (Onishchenko, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010; Popova, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017). Children under the age of 14 accounted for 15–20% of diagnosed cases between 2006 and 2009 (Onishchenko, Reference Kosminkov2007, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010), but their share dropped to 7–16% between 2013 and 2016 (Popova, Reference Popova2014, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017).
Among the infected individuals, the proportion of those residing in urban areas increased from 64% in 2013 to 73% in 2016. Official data showed about 44% of the infected individuals were unemployed and retirees, while students accounted for about 14% (Popova, Reference Popova2014, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017).
According to the origin, meat bought at farmers’ markets was the most common source of infection, in 53–60% of cases, while meat from backyard slaughter for personal consumption was the source of 25–30% of all cases. Meat purchased in retail outlets was a source of infection in about 12–15% of cases (Onishchenko, Reference Kosminkov2007, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010).
Nearly half of all cases of taeniasis occurred due to the consumption of raw or undercooked minced meat, and another 25–35% were due to consumption of barbecued skewered cubed meat (shashlyk). Cured meat was a source of infection in up to 5% of cases (Onishchenko, Reference Kosminkov2007, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010). According to season, cases were registered throughout the year in the whole of RF, generally more often at the end of winter (Onishchenko, Reference Kosminkov2007, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010; Popova, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017). However, when urban areas were singled out, the peak of the infection was at the end of summer and during the fall (Khudyakova, Reference Khudyakova2014).
Occurrence of cysticercosis
Cysticercosis is classified among rare helminthiases in the RF. For example, in 2008, in 43 federal units, 360 cases of rare helminthiases (0.25 per 100,000 population) were registered, of which 200 (55.5%) were cases of clonorchiasis and heartworm disease, while the other 160 included cases of strongyloidiasis, hookworm infection, schistosomiasis, fascioliasis, paragonimiasis, nanophyetiasis, dicrocoeliasis, dipylidiasis, anisakiasis, dioctophymiasis and cysticercosis, without allocation per infection (Rospotrebnadzor, 2009). In 2009, in 44 federal units, 171 cases of rare helminthiases (0.12/100,000) were registered, of which only one case was cysticercosis, in the Krasnoyarsk Territory (Rospotrebnadzor, 2010). Therefore, cysticercosis was registered only sporadically (Rospotrebnadzor, 2009, 2010).
A total of four cases of neurocysticercosis (NC) have been identified in the study period. There were no data on travel history or ethnicity of these patients. In two, the diagnosis was based on clinical manifestations suggesting NC, neuroimaging studies (evidence of cystic lesions showing the scolex, and lesions largely suggesting NC) as well as serological examination (Tumolskaia et al., Reference Ternovykh and Romancheva2002; Ternovykh & Romancheva, Reference Sarbasheva, Bittirova, Atabieva, Bittirov and Bittirov2019). In one case with a fatal outcome involving a farm worker from Tyumen region, the diagnosis was made by post-mortem examination (Kalashnikov et al., 2014). One case was only mentioned in official reports (Bikin Municipal District Official Administration, 2017). In addition to NC cases, a rare case of isolated hepatic cysticercosis (firstly suspected by high-sensitivity ultrasound examination) was recorded in a nine-year-old girl (Figurnov et al., 2002), as well as two cases of ocular cysticercosis, both registered in Moscow, but one originated from the Novosibirsk region whereas the other one was imported from Azerbaijan (Khudyakova, Reference Khudyakova2014).
Occurrence of Taenia solium infection in swine
Data on T. solium infection in pigs in the RF were not plentiful for the analysed period. Official data on the number of infected animals were available only for the years 2006, 2007, 2009 and 2016 (Onishchenko, Reference Kosminkov2007, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010; Gorokhov et al., 2011; Popova, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017). These data, linked to the data on the annual number of slaughtered pigs (Federal State Statistics Service), indicate a great reduction in infection after 2006 (χ2 (df = 3) = 422.351, P < 0.0001) (fig. 5) (Onishchenko, Reference Kosminkov2007, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010; Popova, Reference Popova2014).

Fig. 5. Number of porcine cysticercosis cases per 1,000,000 slaughtered pigs in the Russian Federation (2000–2019) (available official data). (Onishchenko, Reference Kosminkov2007, Reference Popova2010; Gorokhov et al., 2011; Popova, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017).
In the RF, meat inspection is carried out at three checkpoints: at the slaughter line, in meat processing plants and at markets. According to official data, most reports of infected meat originated from meat processing plants, while the lowest number of reports came from slaughter points (table 1). At the slaughter points, there was an obvious decline in the number of infected animals (χ2 = 618.2, P < 0.0001) (table 1). In 2009, 99 cases of cysticercosis in pigs were registered in the Altai, Krasnoyarsk, Perm Territories, Komi Republic, Volgograd, Kirov and Moscow regions (Onishchenko, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010). Data for individual federal units from other years also confirm that the infection was rare and sporadically registered. Between 2011 and 2015, cysticercosis was diagnosed in 0.1% of 2,331,980 slaughtered pigs in the Altai region, but only in 2011 and 2015 (Ponamarev & Luneva, Reference Onishchenko2017). On the other hand, in Karelia, a single case was registered in 2006 and no more cases were detected until the end of the study in 2016 (Rospotrebnadzor Republic of Karelia, 2016). Furthermore, studies have shown no cases of cysticercosis among 201,307 examined pig carcasses in the Ryazan area in 2015 (GVI Ryazan, Reference Figurnov, Churin, Lenshin, Grigorenko, Churina, Figurnova and Soldatkin2015), nor among 896,737 examined pigs in Perm Krai between 2011 and 2015 (Doronin-Dorgelinsky & Sivkova, Reference Doronin-Dorgelinsky and Sivkova2017). Similarly, no cases of cysticercosis were reported either in Novosibirsk between 2006 and 2013 (Amirokov & Zybareva, Reference Amirokov and Zybareva2017) or in the Tula area between 2006 and 2010 (Derzhavina et al., Reference Derzhavina, Boldyreva, Bukreev and Shevelyova2011).
Table 1. Number of infected pigs at inspection checkpoints in 2006, 2007 and 2016 in the Russian Federation.

a Total number, possibly includes uninspected pigs.
No correlation (Pearson's r = −0.2295, P = 0.584) was found between pork production and the frequency of human taeniasis according to federal district (data for 2014 were used in the analysis) (Klimenko et al., Reference Kiriltsov2016).
There are no data on the role of wild boar in the epidemiology of taeniasis in the RF. This role cannot be ruled out, however, since a study of 235 wild boar hunted during the 2007–2016 period in the Trans Baikal region has shown the presence of Cysticercus cellulosae on postmortem examination in 2.1% animals (Kiriltsov, Reference Kalashnikov, Mironov and Zoroastrov2018)
Discussion
In the RF, T. solium infection in humans and animals is mandatory reportable. That is why this study included a relatively small number of published papers, because other papers duplicated data already given in the official reports.
Taenia solium infection is still continuously present in the RF, but with a decrease in the incidence in the 2000–2019 period. The reported decline in the incidence of T. solium taeniasis after 2000 is probably a continuation of a long-term declining trend brought about by the continued implementation of preventive measures since the 1960s, as is the case with the incidence of T. saginata infections (Bobić et al., Reference Bobić, Thomas and Djurković Djaković2018). The reduction in the incidence of infection is seen in porcine cysticercosis too. There are no sufficient data for a similar assessment on cysticercosis in humans, but it certainly does occur rarely.
In the period between 2008 and 2019, taeniasis was registered in 64 out of 85 federal administrative units of the RF. Of these, taeniasis was continuously registered in only six administrative units. The occurrence of sporadic cases in most areas is probably the result of travel or immigration of people from endemic areas or consumption of meat from endemic regions, rather than of autochthonous infection. Such sporadic cases can, of course, be a source of environmental contamination and lead to the development of cysticercosis in humans and pigs, which could lead to the establishment of an autochthonous disease cycle. However, success in the prevention of T. solium infection is also indicated by the fact that the number of administrative units in which taeniasis is registered is declining. The incidence of taeniasis has also decreased in regions where the infection was continuously registered, except for the Komi Republic.
The analysis of the epidemiological characteristics of T. solium infection in the RF must consider its vastness, with a territory that occupies more than 17 million square kilometres, which is more than a tenth of the Earth's land surface. All types of climate, except tropical, are present, and the relief is quite variable, but with plains occupying the largest part of the country (Pushkin State Russian Language Institute, https://www.pushkin.institute). A study in the Republic of Kabardino-Balkaria showed that land elevation has an influence on the prevalence of T. solium infection (Sarbasheva et al., 2012). As the RF population of 145 million includes, in addition to the majority Slavic population (mostly Christians who consume pork), more than 100 ethnic groups (Pushkin State Russian Language Institute, https://www.pushkin.institute), it is expected that there is a wide diversity of lifestyles (customs, religion, diet), which can contribute to differences in the frequency of infection among different areas in the RF. Success in the implementation of preventive measures is influenced by the population's commitment to traditional living habits, but also by their living standard. Differences in the incidence of taeniasis between individual federal districts were not conditioned by differences in population size, however large. However, existing differences in the level of economic development between districts affected the extent of prevention programme implementation – hence, the lower incidence of taeniasis in economically more developed districts (as measured by GDP), and, conversely, the higher incidence in less developed districts. None of these comparisons were possible for cysticercosis due to a small number of cases.
In general, a slightly higher number of cases of taeniasis during the winter period coincides with the traditional time of pig slaughter in most parts of the country. In urban areas, the summer period of excursions and barbecues in nature affects the occurrence of infection, because meat is often procured directly from households in suburban villages or informal local markets where control is lacking (Khudyakova, Reference Khudyakova2014). It is considered that 25–35% of cases can be attributed to skewer consumption alone. The meat purchased at farmers’ markets was the most common source of infection (in 53–60% of cases), while meat from backyard slaughter was a source of 25–30% of all cases (Onishchenko, Reference Kosminkov2007, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010). Consumption of uncontrolled meat from informal markets can also be explained by the social standard of the infected individuals. Most of them were unemployed and pensioners (47%), who usually bought meat from these markets due to lower prices (Popova, Reference Popova2014, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017). Put together, these facts explain the increase in the proportion of urban residents among infected people from 64% in 2013 to 73% in 2016 (Popova, Reference Popova2014, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017). The infection is more often diagnosed in women (Onishchenko, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010; Popova, Reference Popova2014, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017), which may be associated with tasting raw meat (minced or not) for salinity and spiciness during meal preparation. Almost half of all taeniasis cases occur due to the consumption of raw or insufficiently heat-treated minced meat (Onishchenko, Reference Kosminkov2007, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010).
Although official records of animal infection were less accessible and detailed than data on taeniasis in humans, they showed a reduction in the incidence of porcine cysticercosis in the RF. Slaughterhouse data underestimated the actual number of infections, as an additional number of cases were revealed at reinspection on the markets. This can be attributed to the lack of inspection, especially during backyard slaughter, or to the insufficient sensitivity of the visual method of inspection (Dorny et al., Reference Dorny, Phiri, Vercruysse, Gabriel, Willingham, Brandt, Victor, Speybroeck and Berkvens2004). Moreover, repeated examinations would probably improve diagnostic sensitivity, as was already demonstrated in the diagnosis of T. saginata infection in cattle (Kosminkov, Reference Kiselev, Belozerov and Zmushko2010). However, given that the reasons for underestimation are constantly present, the trend of declining infection seems undisputable. The reduction in the number of infected animals in industrial plants where the meat comes from different regions, and where the inspection is always performed and is more thorough, confirms the general reduction in the incidence of pork cysticercosis.
A possible reason for the decline in the infection of pigs in the analysed period may be the increase in the share of pigs originating from industrial farms (from 53.2% in 2003 to 87.8% in 2018) (Klimenko et al., Reference Kiriltsov2016; Zimnyakov, Reference Van Damme, Mwelwa, Mwape, Hobbs, Phiri, Masuku, Zulu, Colston, Willingham and Devleesschauwer2019) where the principles of good production practice are applied more strictly than in backyard or small farms. This is confirmed by the following arguments. Between 1990 and 2000, livestock production had generally declined in the RF, with the number of pigs declining from over 31.5 to about 15.8 million (Klimenko et al., Reference Kiriltsov2016). After 2000, there was a recovery in production, so that in 2018 it amounted to 23.7 million pigs (Klimenko et al., Reference Kiriltsov2016; Zimnyakov, Reference Van Damme, Mwelwa, Mwape, Hobbs, Phiri, Masuku, Zulu, Colston, Willingham and Devleesschauwer2019). Domestic production in 2018 thus met 94.2% (66.8% in 2011) of pork consumption in the RF (almost doubled from 2000 to 2015 from 12 to 23.8 kg per capita per year) (Klimenko et al., Reference Kiriltsov2016). However, this did not result in an increase in the incidence of taeniasis in the RF. Also, the fact that there is no correlation between the occurrence of taeniasis and the number of pigs produced by federal districts confirms the crucial importance of pig farm bio-security.
The reduction of T. solium infection in humans and pigs in the RF confirms the effectiveness of the existing prevention programme. The fact that almost three times as many cases of taeniasis were detected in patients who seek treatment than during mandatory work-related examinations reflects the heightened awareness of symptoms, probably as a result of health education (Onishchenko, Reference Kosminkov2007, Reference Moher, Liberati, Tetzlaff, Altman and The PRISMA Group2010; Popova, Reference Popova2014). Deficiencies in the implementation of control measures somewhat reduce the programme efficiency. The most important weakness probably lies in the insufficient coverage by meat inspection, as well as the low sensitivity of the method (Dorny et al., Reference Dorny, Phiri, Vercruysse, Gabriel, Willingham, Brandt, Victor, Speybroeck and Berkvens2004), since the meat bought in retail outlets can still be a source of infection. Perhaps the sensitivity could be improved if incision of the diaphragm or hind legs were included in the routine examination (Phiri et al., Reference Onishchenko2006; Van Damme et al., 2017). The scope of the inspection is conditioned by infection prevalence – for instance, meat inspection in the European Union, where porcine cysticercosis is extremely rare, does not involve the incision of muscles except in suspect cases (EU Regulation 2019/627, Reference Coral-Almeida, Gabriël, Abatih, Praet, Benitez and Dorny2019). Official reports (Popova, Reference Popova2014, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017) also mention the need to improve sanitary conditions in urban and rural settlements, to develop more efficient wastewater disinfection methods, as well as a better interaction between sanitary, epidemiological and veterinary surveillance experts and the authorities. Further efforts to overcome these problems, as well as to improve pig farming conditions in a sustainable manner, can result in a further decline in the incidence of autochthonous T. solium infection in the RF.
A possible limitation of this study is that prevalence data for T. solium taeniasis were taken on face value from official reports. For instance, there were no data for the Kabardino-Balkaria Republic in the official reports, whereas literature data showed cases, which implies this may be the case in other regions (of the 22) that did not report infection. Another reason for misreporting may be the inadequate specificity of the diagnostic methods – that is, the diagnosis of taeniasis by microscopic examination of faecal samples – because morphological distinction between T. saginata and T. solium is not always reliable in routine examinations. Although official reports account separately for T. saginata and T. solium infections, it needs to be stressed that for some regions (e.g. four in 2016) there were no clear data on diagnostic criteria (Popova, Reference Phiri, Dorny, Gabriel, Willingham, Sikasunge, Siziya and Vercruysse2017). An additional difficulty is that the sensitivity of microscopic examination varies primarily due to mistiming of the sampling, related to intermittent proglottid detachment. Therefore, the sensitivity may vary from 0 to 70%, depending on whether the examination was performed before or after therapy (administration of purgatives) (Rodriguez-Canul et al., Reference Popova1999). Nevertheless, as the examinations in the RF were performed according to the methodological guidelines that have been in place for 60 years, we believe that comparison of data is possible. As for the data on human cysticercosis cases, the exact numbers were not available. For data on cysticercosis in pigs, official reports were presented only for certain years and data are the result of meat inspections using a method of known limited sensitivity. Despite its limitations, however, approximately up to half of the infected animals (Dorny et al., Reference Dorny, Phiri, Vercruysse, Gabriel, Willingham, Brandt, Victor, Speybroeck and Berkvens2004; Phiri et al., Reference Onishchenko2006) can be detected by meat inspection and it is a widely used method worldwide. In the RF, the masseters, heart muscle and tongue are routinely incised, a method less sensitive but the only practical alternative to full necropsy as the method of choice for a definitive diagnosis (WHO, Reference Trevisan, Sotiraki and Laranjo-González2015).
In conclusion, the incidence of T. solium infection in humans and pigs in the RF decreased between 2000 and 2019. It is suspected to be endemic in most of the RF, and veritably endemic areas still exist, indicating that it is a persisting health and economic issue, but of declining importance. However, the proven dependency of the frequency of infection on the economic situation and trends, which are difficult to predict, requires vigilance and additional efforts in the implementation of prevention measures, especially more comprehensive meat control.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0022149X21000432
Financial support
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (contract number 451-03-9/2021-14/200015).
Conflicts of interest
None.
Author contributions
Conceptualization, B.B.; methodology, B.B. and O.Dj.Dj.; systematic review of literature, B.B.; data extraction, B.B., T.Š., J.S., N.B.; data analysis, B.B.; original draft preparation, B.B.; writing – review and editing, I.K., O.Dj.Dj.; visualization, V.Ć.; final review, B.B., I.K., T.Š., J.S., N.B., O.Dj.Dj; supervision, O.Dj.Dj. All authors have read and agreed to the published version of the manuscript.
Data availability
All references found eligible in our literature review are included in the article.








