Introduction
The characteristics of nematode spicules are used widely to distinguish between species, because they vary in shape and size not only between nematode genera but also between congeners (MAFF, 1986; Kaufmann, Reference Kaufmann1996). It is not usually possible to examine and measure worms immediately after their hosts have been culled, because hosts may have been trapped in remote locations, distant from laboratory facilities, and may have to be culled locally and then preserved intact or after separation of body parts. In practical terms, helminths are usually extracted at subsequent laboratory dissection of intestinal tracts or other body organs that have been preserved and stored for varying periods, sometimes for decades or even longer.
The most commonly implemented methods of preservation can be divided into chemical and temperature dependent (freezing at low temperature), often in a saline or similar medium. Moelans et al. (Reference Moelans, ter Hoeve, van Ginkel, ten Kate and van Diest2011) have pointed out that an optimum fixative should allow for detailed morphological analysis, without any marked changes in the target tissue. Nevertheless, each method is known to have its own, distinct effects on tissues (Stoddard, Reference Stoddard and Horie1989), although the precise range of the possible effects on nematode spicules is not known. However, knowledge about the extent of the influence of different preservatives on spicule length is crucial if measurements from different isolates are to be compared, for example, for taxonomic purposes.
Perhaps the most widely used chemical preservative in the past was 10% formalin (10% solution of 100% formalin containing 40% formaldehyde by volume, and hence 4% formaldehyde). Formalin has been widely employed as a biological preservative because it inhibits cellular processes, prevents tissue degradation, preserves tissue architecture and kills pathogens within lesions upon death of the tissue (Eltoum et al., Reference Eltoum, Fredenburgh, Myers and Grizzle2001; Ramos-Vara, Reference Ramos-Vara2005). It also allows long-term storage of material and preserves morphological features well (Moelans et al., Reference Moelans, ter Hoeve, van Ginkel, ten Kate and van Diest2011). Boag (Reference Boag1984) studied the effect of different concentrations of formalin on Trichostrongylus retortaeformis and found that the nematodes were well preserved for up to four months at a concentration as low as 0.78% formalin. However, Fagerholm (Reference Fagerholm1979) observed shrinkage in the length of Eustrongylides tubifex in formalin. Both studies were on entire adult worms, the bodies of which are softer than highly sclerotized spicules, and are, therefore, more likely to react to preservatives. Importantly, formalin also damages DNA sufficiently to complicate, and in some cases totally impair, the recovery of intact DNA from formalin-preserved tissues (Sepp et al., Reference Sepp, Szabó, Uda and Sakamoto1994; Frank et al., Reference Frank, Svoboda-Newman and Hsi1996). It is, therefore, a useful preservative if specimens are to be available for morphological study and morphometric assessment, but not for molecular genetic analysis. Moreover, formaldehyde is toxic and carcinogenic, and is used less frequently now than in the past (Squire & Cameron, Reference Squire and Cameron1984; Morgan, Reference Morgan1997; Dimenstein, Reference Dimenstein2009; Songur et al., Reference Songur, Ozen and Sarsilmaz2010; Elshaer & Mahmoud, Reference Elshaer and Mahmoud2017; Agency for Toxic Substances and Disease Registry, 2021).
Another widely used chemical preservative is alcohol (usually ethanol, but also, to a lesser extent, methanol), of which varying concentrations may be exploited to preserve biological specimens. Ethanol is a powerful dehydrating agent that prevents deterioration of tissues, but causes various degrees of shrinkage and distortion of morphology, depending on concentration and tissue type (Naem et al., Reference Naem, Pagan and Nadler2010). Loss of water molecules occurs from all tissues and in the case of nematodes also from the pseudocoelomic cavity (Naem et al., Reference Naem, Pagan and Nadler2010). This dehydrating effect of ethanol helps to provide excellent and long-term protection of DNA by denaturation of endogenous proteins that degrade DNA (Dessauer et al., Reference Dessauer, Cole, Hafner, Hillis, Moritz and Mable1996; Flournoy et al., Reference Flournoy, Adam and Pandy1996).
Freezing (commonly employed temperatures are −20, −70 and −80°C) provides another excellent method for storing biological specimens as it preserves key macromolecular resources including DNA, RNA, as well as proteins. Storing specimens at low temperature keeps them intact, preserving a sample's integrity and viability over a long period. However, freezing can also damage tissues through formation of ice crystals, and result in distortion of tissue morphology (Dessauer et al., Reference Dessauer, Cole, Hafner, Hillis, Moritz and Mable1996).
The effects of formalin, ethanol and freezing have been compared on various animal species in previous studies (Glenn & Mathias, Reference Glenn and Mathias1987; Hjörleifsson & Klein-MacPhee, Reference Hjörleifsson and KIein-MacPhee1992; Jawad, Reference Jawad2003; Qureshi et al., Reference Qureshi, Saher, Niazi and Gondal2008). However, while no work has been carried out specifically to compare the effects of these preservatives on the spicule length of Heligmosomoides bakeri, more generally it has been reported that the spicules of nematodes are not distorted by fixation (Croll & Matthews, Reference Croll and Matthews1977). In an earlier paper, we drew attention to the range of reported values for the length of the spicules of Heligmosomoides polygyrus and H. bakeri (Musah-Eroje et al., Reference Musah-Eroje, Burton and Behnke2021), which may have been attributable partially to differences between studies in the methods used for preservation of worms prior to measurement of spicules. Therefore, we report here the results of experiments in which we assessed and compared the effects of conventionally used preservatives on the spicule length of H. bakeri worms, in order to clarify the degree to which variation in spicule length can be explained by different methods of preservation.
Materials and methods
Parasites, mice and spicules
In this paper we refer to H. bakeri (previously known as Nematospiroides dubius, H. polygyrus and H. polygyrus bakeri), as the parasite maintained in laboratory mice (Cable et al., Reference Cable, Harris, Lewis and Behnke2006; Behnke & Harris, Reference Behnke and Harris2010). We used H. bakeri since this species is easily maintained in the laboratory and infection of laboratory mice at a single time point generates sufficient worms of an identical age (Ehrenford, Reference Ehrenford1954; Bartlett and Ball, Reference Bartlett and Ball1974). Male worms were obtained from BKW strain laboratory mice (purchased from Bantin and Kingman Universal Ltd., Grimston, Aldbrough, Hull, North Humberside, UK), 21 days after infection with 250 larvae. The mice were housed under standard conditions in individually ventilated cages in which water and standard mouse chow were provided ad libitum, and were killed humanely using the approved Schedule 1 method of exposure to an increasing concentration of carbon dioxide gas in an enclosed chamber. Their small intestines were excised and placed into Petri dishes containing Hanks’ balanced salt solution (HBSS) on an incubator maintained at 37°C. After one hour, worms were seen to have migrated into the solution, and were carefully transferred with fine-tipped forceps into tubes containing one of the fixatives specified below.
The spicules of H. bakeri are filiform, slender, slightly expanded at their roots and with a fine point at their distal end. Each male has two spicules of almost the same length and same shape, and where possible each of the two was measured in randomly chosen worms (see Musah-Eroje et al. (Reference Musah-Eroje, Burton and Behnke2021) for photographic images and Baylis (Reference Baylis1926) for description of spicules of the closely related species H. polygyrus).
Experimental procedures
In Experiment 1, five different treatments were compared: 70% ethanol at room temperature, 70% ethanol plus freezing at −80°C, 10% formalin at room temperature, 10% formalin plus freezing at −80°C and freezing at −80°C in HBSS. Room temperature was approximately 18–21°C. Twenty spicules were measured from each of the five preservation methods, at each of the two time intervals after extraction from mice – one week of preservation (n = 100) and one month of preservation (n = 100). Frozen specimens were thawed. Worms were carefully picked with fine-tipped forceps, placed on a clean slide and were cleared in one to two drops of lactic acid. They were then photographed, and the spicules were measured subsequently from the photographs using Image J software as previously described (Musah-Eroje et al., Reference Musah-Eroje, Burton and Behnke2021). The second experiment was carried out similarly except that the time intervals for measurement of spicules were one and four months from autopsy of donor mice, and in this experiment, 80% ethanol was used. In Experiment 1, we also included spicules from worms maintained overnight at 4°C in HBSS and then measured on the day following extraction at autopsy.
Statistical analysis
Data were stored in Excel and analysed in R version 4.1.0. (R Core Development Team, the R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/). Summary statistics are presented as mean spicule lengths with standard errors of the mean (SEM), and range of values. For Experiment 1, we fitted a general linear model with Gaussian errors, comprising three explanatory factors, treatment (three levels, HBSS, 70% ethanol and 10% formalin), time (two levels, one week and one month after extraction) and freezing (two levels, no freezing or freezing at −80°C). Experiment 2 was analysed similarly, except that 80% ethanol was used and the time intervals were one and four months after extraction of worms from donor mice. Full factorial models were simplified by backwards election, using deletion of terms beginning with the highest order interaction by comparing models with or without that interaction. Minimum sufficient models were then fitted (all significant interactions and main effects plus any main effects that featured in interactions), and the process was repeated to obtain values for changes in deviance, test statistics (F) and probabilities. The percentage of deviance accounted for by each significant main effect or interaction was calculated as recommended by Xu (Reference Xu2003) and reported by Behnke et al. (Reference Behnke, Bajer and Harris2008). The goodness of fit of models was assessed by checking that residuals conformed to the Gaussian distribution.
Results
Experiment 1
The results of the first experiment are shown in table 1. The mean spicule length of fresh worms derived within 24 h of extraction from mice (0.524 ± 0.005 mm) was much in line with our published values for spicule length (0.518 ± 0.003 mm for 21-day-old worms; Musah-Eroje et al., Reference Musah-Eroje, Burton and Behnke2021). These values were excluded from the fitted statistical model, because for obvious reasons fresh worms could only be utilized within a day of extraction from donor mice. The mean length of spicules, all treatments and times combined, was 0.526 ± 0.0015 (n = 200). The main effect of freezing was not significant (F 1, 195 = 0.03, P = 0.87), nor were any interactions incorporating freezing significant, although the most marked shrinkage in mean length was in worms that had been frozen at −80°C in HBSS (table 1). For analysis of the remaining data, the minimum sufficient model only included one significant combination (two-way interaction, treatment × time, F 2, 194 = 9.7, P < 0.0001, accounting for 9.1% of deviance), neither of the two main effects showing significance (for treatment F 2, 196 = 1.65, P = 0.20; for time F 1, 196 = 0.27, P = 0.6). The interaction arose because in some combinations, the mean length of spicules was marginally longer after four weeks (ethanol + freezing, and formalin at room temperature), and in others shorter (HBSS + freezing, and formalin + freezing), while in one case there was virtually no change in length (ethanol at room temperature, just 1.4 μm). The largest discrepancy in mean lengths between treatments in week 1 was 4.95% (between worms frozen in HBSS and those in formalin at room temperature) and in week 4, 2.63% (between worms in formalin at room temperature and those frozen in HBSS).
Table 1. Experiment 1: effect of preservatives on the range and mean spicule length after periods of storage for one or four weeks in duration.
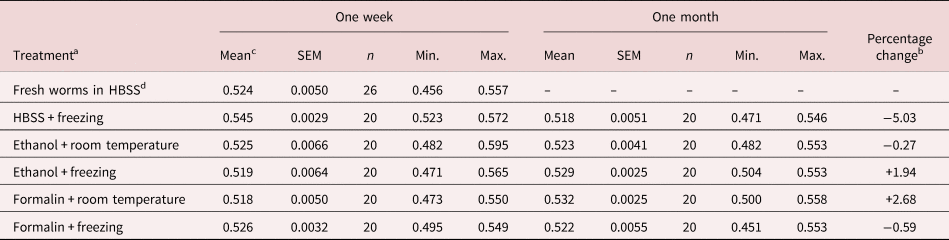
SEM, standard error of the mean.
a Worms were extracted in HBSS, and spicules were measured on fresh worms, after preservation in 70% ethanol or 10% formalin at room temperature or at −80°C. A different batch of spicules was measured at one week and one month.
b Percentage change is calculated as the difference between length in week 1 and week 4, expressed as a percentage of length in week 1, using values to five decimal points.
c Values for mean length, SEM, Min. (minimum length) and Max. (maximum length) are in mm.
d Values for fresh worms were derived within 24 h of extraction from mice.
Experiment 2
The results of the second experiment are shown in table 2. The mean length of spicules, all treatments and times combined, was 0.527 ± 0.0012 (n = 352). As in the first experiment, the main effect of freezing was not significant (F 1, 347 = 0.009, P = 0.92), nor were any interactions incorporating freezing significant. For the remaining data, the minimum sufficient model included two significant main effects and one two-way interaction. There was a highly significant overall shortening of spicule length over time (F 1, 348 = 33.4, P < 0.0001, accounting for 8.8% of deviance; mean lengths after one and four months were 0.534 ± 0.0017 mm (n = 186) and 0.520 ± 0.0016 mm (n = 166), respectively constituting a reduction in mean length over time of 2.529%). The most marked shrinkage in mean length of spicules over time in this experiment was for worms kept in 80% ethanol at room temperature (table 2). The overall difference between treatment by freezing, ethanol or formalin showed only marginal significance (main effect of treatment, F 2, 348 = 3.073, P = 0.048, accounting for 1.7% of deviance). The overall mean lengths for spicules in HBSS, 80% ethanol and 10% formalin were 0.527 ± 0.0027 (n = 74), 0.531 ± 0.001 (n = 148) and 0.524 ± 0.0021 (n = 130), respectively. The largest discrepancy in mean lengths between treatments in month 1 was 4.32% (between worms in ethanol and formalin, both at room temperature) and in month 4, 2.33% (between worms frozen in HBSS and those in formalin at room temperature).
Table 2. Experiment 2: effect of preservatives on the range and mean spicule length after periods of storage for one or four months in duration.
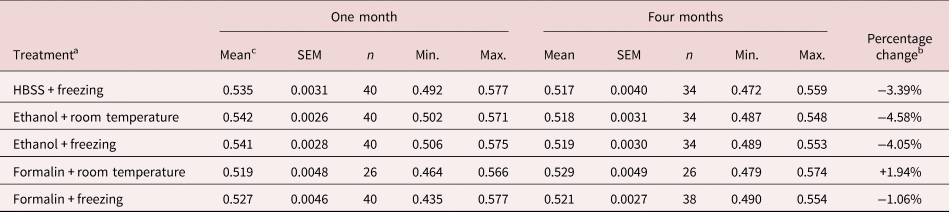
SEM, standard error of the mean.
a Worms were extracted in HBSS, and spicules were measured after preservation in 80% ethanol or 10% formalin at room temperature or at −80°C. A different batch of spicules was measured at one and at four months.
b Percentage change is calculated as the difference between length in month 1 and month 4, expressed as a percentage of length in month 1, using values to five decimal points.
c Values for mean length, SEM, Min. (minimum length) and Max. (maximum length) are in mm.
The two-way interaction, treatment × time (F 2, 346 = 11.97, P < 0.0001, accounting for 6.5% of deviance), arose because the degree of shrinkage of spicule length over time varied significantly between treatments (table 2). Spicules in 10% formalin (frozen and at room temperature combined) showed a marginal increase in length over time (+0.13%), but those in HBSS (frozen) showed 3.39% shrinkage in mean length and those in 80% ethanol (frozen and at room temperature combined) showed a 4.32% reduction in mean length.
Discussion
Given the known effects of freezing, ethanol (Naem et al., Reference Naem, Pagan and Nadler2010) and formalin (Fagerholm, Reference Fagerholm1979; Boag, Reference Boag1984; Fox, Reference Fox1996) on biological tissues, it was reassuring to find in the present study that the spicules of H. bakeri showed only modest changes in length in respect of the different treatments and over time. Our data showed that while there were some differences between treatments and in the mean lengths recorded over time, these were relatively minor and did not exceed 5.03% shrinkage in length. This is an important conclusion from our work because the intact bodies of rodents or their intestinal tracts, as well as the parasitic worms, are often preserved for long periods before intricate examination of the worms is possible. On a broader front, this is also the case for other surveys of host–parasite combinations, and generally in the fields of parasite ecology and epidemiology.
In the first experiment, over the shorter time interval between first and second recordings of length, despite some inconsistencies between treatments, none differed significantly in their effects on spicule length and there were no significant changes with time. Moreover, the average length of the spicules in all the treatments combined was very similar to that recorded for worms that had been kept overnight at 4°C. The second experiment was conducted over a longer timeframe, utilizing 80% rather than 70% ethanol, and with larger sample sizes, but overall the mean length of all samples combined was virtually identical with that recorded in Experiment 1 (0.527 vs. 0.526 mm, respectively), indicating good concordance of measurements between the two experiments. In contrast to the first experiment, here we recorded an overall decline in mean length of spicules (2.529%) over the longer period used in this experiment. This was mostly attributable to ethanol treatment (a reduction in mean length of 4.318%) and very similar whether the worms in ethanol had been frozen or kept at room temperature. The loss of water inevitably results in some shrinkage of tissues. Hence, we can speculate that loss of water molecules from the protein complexes that constitute spicules may explain the shrinkage observed in Experiment 2. Spicules have similar chemical composition to the cuticle, which is mainly composed of water and proteins (Bird & Bird, Reference Bird and Bird1991). Naem et al. (Reference Naem, Pagan and Nadler2010) noted that preservation of nematodes in high concentrations of alcohol resulted in structural dehydration and artefacts which may include shrinkages and distortion of body surface, thereby affecting the exactness of features subject to morphometric analysis. However, while ethanol is not ideal for long-term storage of specimens for subsequent morphometric assessment, ethanol is an excellent preservative for DNA and is widely used for material that is required subsequently for molecular analysis.
Although at one month in Experiment 2, the mean length of spicules in 10% formalin was shorter than those preserved in 80% ethanol or frozen in HBSS, perhaps unexpectedly, there was no major change over the following three months in this preservative (increase of 1.94% at room temperature and shrinkage of 1.06% at −80°C). If there was any shrinkage of spicule length in Experiment 2, it may have occurred by the first month, although the mean spicule length was very similar to mean lengths recorded in fresh specimens and spicules measured after just a week in Experiment 1. Formaldehyde is known to cause shrinkage of biological tissues. For example, Fox (Reference Fox1996) reported that when herring larvae were placed directly into formaldehyde, shrinkages occurred up to 30 days but the changes in the length were not statistically significant after 15 days. Fagerholm (1976) also noted shrinkage in the length of Eustrongylides tubifex as the nematodes came into contact with formalin. Shrinkage of tissues in preservatives has been attributed to a consequence of a combination of chemical fixation of the tissue and osmotic imbalance between the specimen and the preservative medium (Ahlstrom, Reference Ahlstrom and Steedman1976). Boag (Reference Boag1984) recommended that gastrointestinal nematodes should be stored in a low concentration of formalin (1%), since low concentrations of formalin do not have a deleterious effect on biological specimens. One of the disadvantages of formalin is that it damages DNA. Hence, it may not represent a suitable preservative for samples that also require molecular analysis for taxonomic and systematic purposes.
HBSS was used in the current experiments because it is neutral (PH 7.2) and high in ion concentration, thus helping to maintain osmotic balance between the specimen (Sultana et al., Reference Sultana, Nikaido, Asafujjoha, Tagami and Matin2006). However, freezing worms at −80°C in HBSS alone, or in ethanol or formalin had no significant effect on spicule length, although in HBSS we recorded a 3.39% shrinkage in mean spicule length over the three-month interval between measurements in Experiment 2. Mean spicule length here was longer than the 0.524 mm for fresh worms in Experiment 1, perhaps suggesting some initial stretching of spicules in frozen HBSS and also in ethanol (both at room temperature and frozen) during the first month of preservation. Any detected stretching of nematodes in different fixatives might be due to the relaxation of the longitudinally orientated somatic muscle cells in combination with the stretching potential of the cuticle, and also the internal pressure exerted by the pseudocoelomic fluid as they come into contact with fixatives (Fagerholm, Reference Fagerholm1979). All these effects may possibly have a knock-on effect on spicule length because modified muscle cells from the body wall are attached to the spicules in their sheaths (Crofton, Reference Crofton1966).
The key conclusion from this study is that while whole nematodes can shrivel and shrink in preservatives, making many measurements unreliable, it seems that spicule lengths are very little changed by preservation techniques over time, and, therefore, spicule length remains as a reliable taxonomic character. Nevertheless, preservatives cause some modest variation in the length of spicules, and whether shrinkage or expansion, the effect is to some extent time-dependent, although our data are drawn from two separate experiments that exploited different time intervals, and different concentrations of ethanol. Overall variation in spicule length was very limited, accounting for no more than 5.03% change in length over time and 4.95% between treatments at any of the four periods for assessment. It is highly desirable, therefore, that microscopic examination of nematodes should be carried out on fresh specimens whenever possible, although in most cases this is unlikely to be a practical solution. Samples usually need to be stored for subsequent laboratory scrutiny. Thus, it is important to keep them in preservative for only a short period of time before examination. Nematodes that have been frozen in ethanol or in HBSS are optimal for subsequent molecular systematic studies, but the most stable preservative for spicule length in the current study was 10% formalin, whether frozen or just kept at room temperature.
Acknowledgement
We thank Ann Lowe for assistance with laboratory work.
Financial support
This work was supported by a scholarship from the University of Nottingham (M.M.E.).
Conflicts of interest
None.
Ethical standards
All animal procedures were carried out under UK Home Office licence number 40/3138 and under the regulations of the Animals (Scientific Procedures) Act 1986. Maintenance of animals conformed to local and Home Office Code of Practice (ISBN 9781474112390).
Author contributions
J.M.B. conceived and designed the study, L.B. and M.M.E. carried out the laboratory work. All authors contributed to the analysis of the data, preparation of the manuscript and all approved the submitted version.






