Introduction
Human cystic echinococcosis (CE), a zoonosis caused by larval forms of the tapeworm Echinococcus granulosus, is a public health problem in endemic areas (Dakkak, Reference Dakkak2010). The domestic strain is endemic in many sheep-rearing areas, particularly in the Mediterranean and Balkan countries, South America, Africa and the former Soviet Union (Dakkak, Reference Dakkak2010; Djuricic et al., Reference Djuricic, Grebeldinger, Kafka, Djan, Vukadin and Vasiljevic2010). Human infection is mediated via the faecal–oral route by consuming food or water contaminated with tapeworm eggs from the faeces of an infected host, usually dogs (Dakkak, Reference Dakkak2010).
In primary CE, after oral ingestion of E. granulosus eggs, forms of the parasite (metacestodes) may develop in almost any organ. Secondary CE is usually the result of spontaneous or trauma/surgery-induced cyst rupture, causing dissemination of the released protoscolices and the development of cysts in many organs. Approximately 40–80% of patients with primary CE have single-organ involvement and harbour a solitary cyst (Pawlowski et al., Reference Pawlowski, Eckert, Vuitton, Ammann, Kern, Craig, Dar, De Rosa, Filice, Gottstein, Grimm, Macpherson, Sato, Todorov, Uchino, von Siner, Wen, Eckert, Gemmell, Meslin and Pawlowski2001). The most common site of infection is the liver (55–70%), followed by the lungs (18–35%) (Khanfar, Reference Khanfar2014). However, 10% of cysts can be found anywhere in the body, including the spleen (6%), heart (2%), kidney (2%) and brain (<2%) (Gottstein, Reference Gottstein2000). Although cysts can grow at a rate of 1–5 cm/year, up to 60% of liver CEs are asymptomatic, even into advanced age (Pakala et al., Reference Pakala, Molina and Wu2016). Hydatid disease is frequently discovered by accident, but it should be considered routinely in the differential diagnosis, especially in endemic regions (Gottstein, Reference Gottstein2000; Pakala et al., Reference Pakala, Molina and Wu2016).
According to the World Health Organization (WHO), E. granulosus is endemic in areas of South America, Eastern Europe, Russia, the Middle East and China, where human incidence rates are as high as 50/100,000 persons/year (Brunetti et al., Reference Brunetti, Kern and Vuitton2010). In the Mediterranean region, the annual incidence of human CE varies from 4 to 8/100,000 persons/year (Eckert et al., Reference Eckert, Schantz, Gasser, Torgerson, Bessonov, Movsessian, Thakur, Grimm, Nikogossian, Eckert, Gemmell, Meslin and Pawlowski2001). In recent years, some of the Balkan countries, such as Romania, Bulgaria and Greece, have reported increasing rates of CE (Sotiraki et al., Reference Sotiraki, Himonas and Korkoliakou2003). Factors contributing to the maintenance of CE in the Balkan region include: (1) rural families living in close proximity to their livestock; (2) the large number of stray and free-roaming dogs; (3) small abattoirs lacking appropriate facilities; (4) home slaughter and consumption of non-inspected meat, with viscera often accessible to dogs; (5) uncontrolled movement and commerce of animals and animal products; and (6) lower educational levels, influence of tradition and prejudices (Sotiraki et al., Reference Sotiraki, Himonas and Korkoliakou2003; Djuricic et al., Reference Djuricic, Grebeldinger, Kafka, Djan, Vukadin and Vasiljevic2010). The annual average incidence of new cases of human CE countrywide has not been documented well in many regions, including Serbia. CE was endemic in former Yugoslavia, with an incidence of 3.7 (1969) and 4.7 (1985) per 100,000 population; hyperendemic regions with an incidence of 12 per 100,000 population include Dalmatia, Herzegovina, Montenegro, eastern Serbia, Sandzak, Kosovo and Macedonia (Petrovic et al., Reference Petrovic, Dugalic and Popovic1989; Djuricic et al., Reference Djuricic, Grebeldinger, Kafka, Djan, Vukadin and Vasiljevic2010). The diagnosis of CE is complex and may entail various steps: (1) suspicion on clinical grounds or upon screening; (2) confirmation by imaging techniques and identification of cyst structures; (3) confirmation by detection of specific antibodies (Ab) with immunodiagnostic tests; and (4) in doubtful cases, diagnostic puncture may be considered if it is not contraindicated (Pawlowski et al., Reference Pawlowski, Eckert, Vuitton, Ammann, Kern, Craig, Dar, De Rosa, Filice, Gottstein, Grimm, Macpherson, Sato, Todorov, Uchino, von Siner, Wen, Eckert, Gemmell, Meslin and Pawlowski2001; Pakala et al., Reference Pakala, Molina and Wu2016).
The treatment of choice for hepatic hydatid disease is surgery. Independently of the type of surgical procedure being performed, the basic goal during cyst removal is to avoid abdominal contamination by hydatid material (Craig et al., Reference Craig, Rogan, Allan, Gillespie and Hawkey1995). Information about the viability of protoscolices is essential for the prognosis of the disease and follow-up of the patient. Pre-operative use of antihelminthic drugs, such as albendazole, may prevent relapses in cases of both multiple and large cysts (Craig et al., Reference Craig, Rogan, Allan, Gillespie and Hawkey1995).
CE influences the human population particularly in developing countries and takes a significant toll on global livestock production, with losses of as much as US$2 billion annually (Brunetti et al., Reference Brunetti, Kern and Vuitton2010).
We conducted this case-series study to evaluate the correlation between seropositivity, socio-epidemiological data, pre-operative treatment with albendazole and viability of protoscolices in human hepatic hydatid cysts.
Materials and methods
Study design and patients
The study was designed as a prospective series of cases, and was carried out on 38 consecutive patients (28 adults and 10 children) with liver E. granulosus, treated surgically from April 2014 to May 2015 at the Department of Surgery of the Clinical Centre of Serbia and University Children's Hospital, Belgrade, Serbia. A questionnaire was completed for each patient and venous blood samples were taken for anti-E. granulosus Ab testing. Epidemiological information, related to echinococcus transmission, was collected. Thirty-eight epidemiological questions were asked: age, gender, socio-geographical status, presence of symptoms, contact with dogs, type and number of dogs, feeding dogs with livestock viscera, place of slaughter of livestock, etc. The following clinical variables were also considered: diameter of the cysts, the number of cysts and pre-operative treatment with albendazole. Pre-operative treatment with albendazole at a dosage of 10 mg/kg per day was given to all ten children and two adult patients for 4 weeks.
Cyst processing and viability testing
Thirty-eight samples from the surgery departments were examined in the parasitology laboratory. Samples included part of the cyst wall, daughter cysts, content of the cyst or aspirated fluid from the cyst. The diagnosis of hydatidosis was confirmed by the presence of protoscolices or brood capsules. In addition, the presence of rostellar hooks indicated that protoscolices were present. If protoscolices were present, viability testing was performed.
Viability was assessed by direct observation under a light microscope. Positivity of the test was considered in accordance with the previously published protocol (Manterola et al., Reference Manterola, Mansilla and Fonseca2005). The degree of viability was calculated according to previously published criteria (Craig et al., Reference Craig, Rogan, Allan, Gillespie and Hawkey1995).
Serology testing
About 5 ml of venous blood was taken from each patient. For IgG Ab testing by enzyme-linked immunoabsorbent assay (ELISA; R-Biopharm, Germany), antigen B (AgB) from hydatid fluid was used as the antigen. The results were evaluated semi-quantitatively by calculating the ratio of the extinction value of the patient's sample to the cut-off extinction value. Samples with a reading equal to or greater than the threshold cut-off value were considered to be positive (≥0.9).
Statistical analyses
Descriptive statistics were used in the calculation of medians, averages and standard deviation. Analytical statistics were used for the comparison of continuous variables, applying the chi-squared test.
Results
Socio-demographic data
During the study period, 38 patients were treated surgically and all patients (38/38, 100%) had liver CE, with a variable number of cysts. Of 28 adult patients, two had secondary echinococcosis. Most of the patients (36/38, 94.7%) lived in central Serbia; 6/38 (15.8%) lived and worked on family farms, raising pigs (4/6, 66.7%) and sheep (2/6, 33.3%). These patients (6/38, 15.8%) stated that they would usually feed viscera and some other entrails to their dogs and other dogs in the neighbourhood. In addition, seven patients living in apartments in urban areas declared that they often visited relatives in the countryside, who actively bred and slaughtered livestock at home and fed viscera to their dogs. For the purpose of the study, we considered this group as a peri-urban population. Socio-epidemiological data are presented in table 1.
Table 1. Comparison of socio-epidemiological parameters between adults and children with hepatic Echinococcus granulosus cysts.
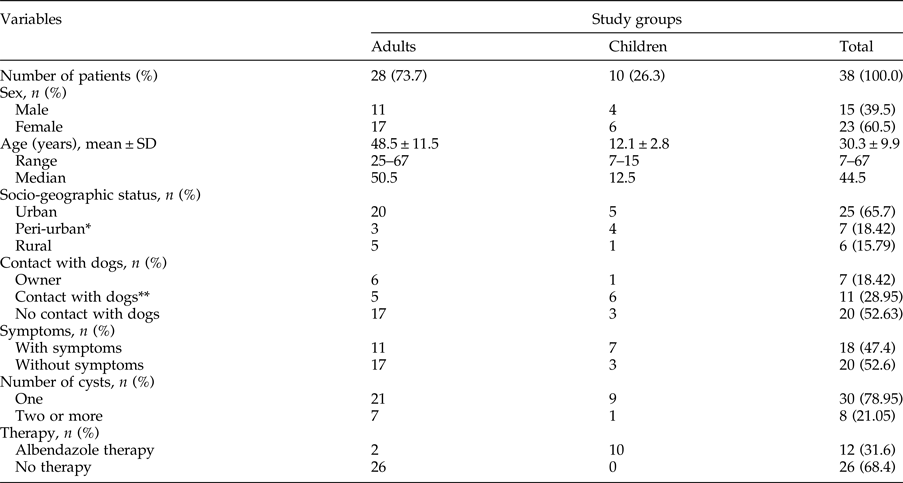
* Patients who live in the city and often visit relatives who rear livestock on their farms and own dogs.
** Contact with dogs in the neighbourhood and/or frequent visits to households with dogs.
In terms of symptoms, 52.6% patients (20/38) were symptomless and the disease was detected incidentally by abdominal ultrasound examination. Slight symptoms were present in 18/38 patients, most frequently described as non-specific right upper abdominal pain during activity and/or at rest, followed by exhaustion, nausea, loss of appetite, further accompanied by asthma-like symptoms in three cases. The average diameter of the cysts in patients who presented symptoms was 5.1 ± 1.5 cm, compared with an average diameter of 4.5 ± 2.2 cm in asymptomatic patients (fig. 1).
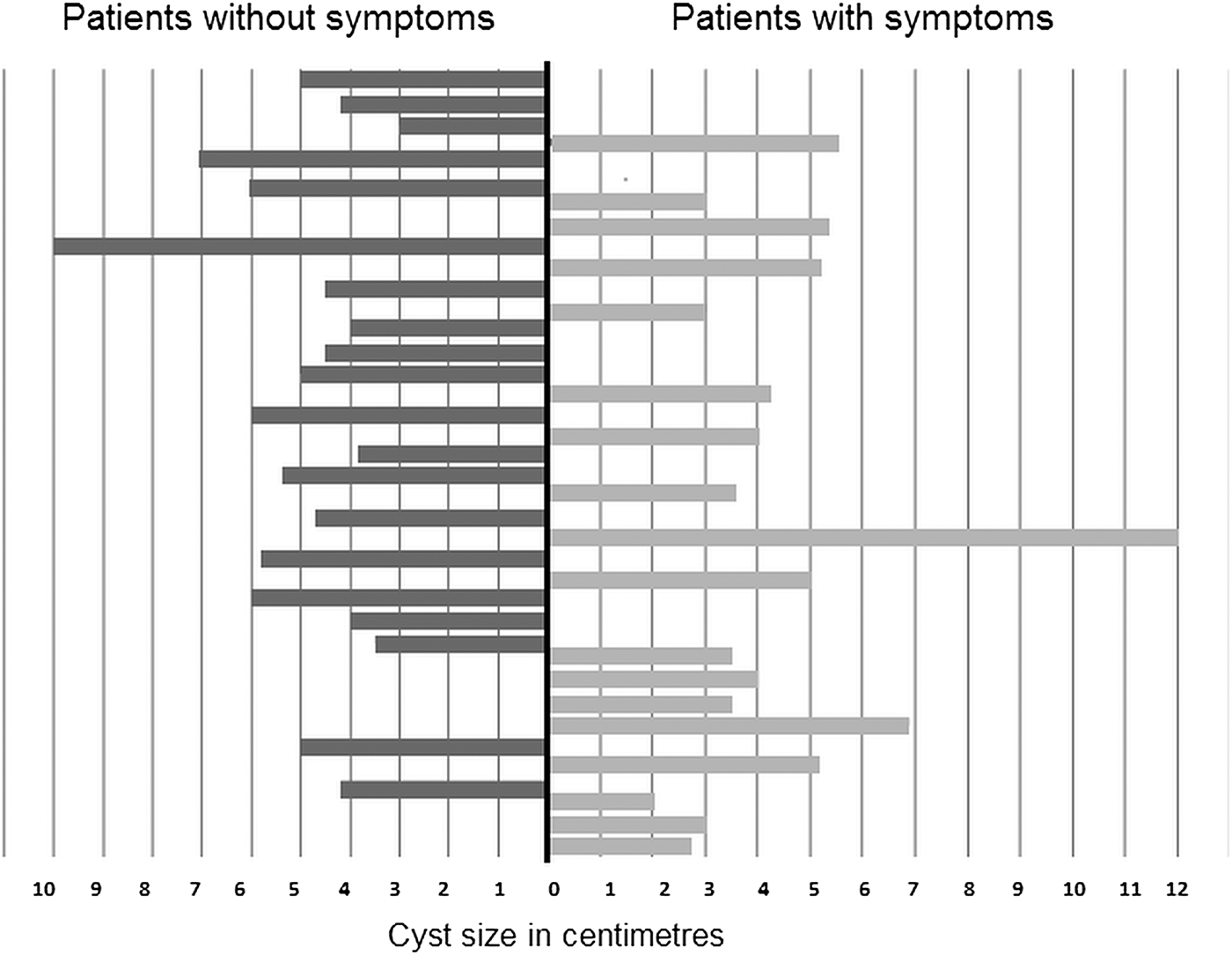
Fig. 1. The cyst diameter in two groups of patients: with and without symptoms.
The viability test was positive in 30 (79%) samples; among eight non-viable cysts, 38% (3/8) were calcified, 25% (2/8) were filled with pus flakes (abscessed cysts) and 38% (3/8) were filled with clear fluid without the presence of protoscolices or rostellar hooks.
Among 12 patients who underwent pre-operative albendazole treatment (10 mg/kg per day), the viability test was positive in 11 (91.6%). Of all 10 children who were on the treatment, the viability test was negative only in one case (10%). Of two adult patients who underwent pre-operative albendazole treatment, one had a viable unilocular cyst and the other had 16 cysts, some of which were calcified and others were viable. In addition, the viability test was positive in 73% (19/26) of cysts from the patients who did not receive pre-operative medical treatment. The association between viability testing and pre-operative albendazole treatment is shown in table 2.
Table 2. Association of viability testing, seropositivity and pre-operative albendazole treatment.
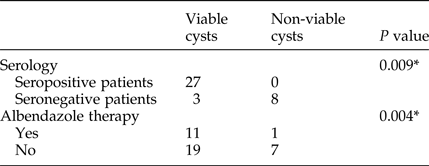
* Chi-squared test.
Serology testing revealed a higher number of seropositive results in patients with viable cysts. The total number of patients with positive serology was 27/38 (71%) (table 2).
Discussion
The increased emigration of populations from endemic areas where prevalence rates are 5–10%, and the relatively quiescent clinical course of CE, pose challenges for accurate and timely diagnoses of CE (Brunetti et al., Reference Brunetti, Kern and Vuitton2010). The global picture of the current epidemiological situation regarding CE remains incomplete because of the lack of well-documented data from many countries. A high prevalence of parasites has been found in parts of Eurasia (Mediterranean region, Russian Federation and adjacent independent states, and China), Africa (southern and north-eastern regions), Australia and South America (Wahlers et al., Reference Wahlers, Menezes, Roming, Kern and Grobusch2013). In some European countries and regions, the annual incidences of hospital cases of human CE vary from 1 to 8 per 100,000 persons (Eckert & Deplazes, Reference Eckert and Deplazes2004). As reported by Seimenis (Reference Seimenis2003), the highest incidence and/or prevalence of CE in humans in the Mediterranean region was found in Italy (8.00/100,000; 1990–1999), followed by Bulgaria (7.42/100,000; 1997–2000) and Greece (3.5–1.22/100,000; 1989–1998). Nonetheless, the epidemiology of CE in Serbia has not been investigated in the past three decades, despite the findings that CE was one of the most commonly diagnosed parasitic zoonoses in 2000–2005 (Antonijevic et al., Reference Antonijevic, Madle-Samardzija, Turkulov, Canak, Gavrancic and Petrovic-Milosevic2007).
In 2014, there were 806 newly diagnosed cases of echinococcosis in the European Union. The incidence rate of echinococcosis (E. granulosis and E. multilocularis) in 2014 and 2015 was 0.18/100,000. From 2008 to 2014, the epidemiological trend of echinococcosis was stable, although it varied depending on the Echinococcus species. In 2015, in the Republic of Serbia, 52 people were diagnosed with echinococcosis, with an incidence rate of 0.73/100,000, the highest incidence rate between 2002 and 2015 (www.batut.org.rs).
The mean age of the adult patients in our study was 48.5 ± 11.5 years, and 12.1 ± 2.8 years for children. Consistent with our findings, a review article on CE in neighbouring Greece reported the highest surgical incidence among adult patients in those aged 40–69 years (Sotiraki et al., Reference Sotiraki, Himonas and Korkoliakou2003). In contrast to that study, which found a higher incidence of CE in the male population, we observed that the number of female patients (60.5%) slightly exceeded the number of males (39.5%).
Consistent with the characteristics of the parasite life cycle, CE is generally considered to be a disease of the rural environment. This was supported by numerous studies demonstrating a predominance of CE patients in rural populations (Altintas, Reference Altintas2003; Schantz et al., Reference Schantz, Wang, Qui, Liu, Saito, Emshoff, Ito, Roberts and Delker2003; Larrieu et al., Reference Larrieu, Del Caprio, Salvitti, Mercapide, Sustersic, Panomarenko, Costa, Bigatti, Labanchi, Herrero, Cantoni, Perez and Odriozola2004; Zanini et al., Reference Zanini, Suarez, Perez and Elissondo2009). In contrast, only a few studies reported more CE patients from urban areas than rural areas, and some studies did not find a significant difference between urban and rural settlements (Ok et al., Reference Ok, Okzol, Kilimcioglu, Dinc, Bayindir, Ostan, Pabuscu, Ozcan, Korkmaz, Coskun, Yuksel and Girginkardeseler2007; Dopchiz et al., Reference Dopchiz, Elissondo, Andresiuk, Maiorini, Gutierrez, Muzulin, Rosenzvit, Lavallen and Denegri2009). In our study, a significantly higher number of cases of CE was found in patients living in urban than in rural and peri-urban areas. Moreover, we found a significantly higher number of patients who reported not having any contact with dogs. In a case of human infection, contamination of the living surroundings with eggs of the parasite is one of the principal ecological determinants of parasitic disease. A large number of stray dogs inhabit urban and peri-urban settlements, presenting a continuous problem in many parts of Serbia. In support, an unpublished study showed that in some parts of eastern Serbia, 30–70% of dogs were found to be infected with E. granulosus, similar to an investigation in Peru, where one-third of dogs were found to be infected with adult Echinococcus tapeworms (Moro et al., Reference Moro, Cavero, Tambini, Briceno, Jimenez and Cabrera2008). Ingestion of E. granulosus eggs via contaminated food and water appears to be the primary source of the infection (Pakala et al., Reference Pakala, Molina and Wu2016).
It was reported previously that liver CE could be asymptomatic in 38–98.5% of patients (Larrieu et al., Reference Larrieu, Del Caprio, Salvitti, Mercapide, Sustersic, Panomarenko, Costa, Bigatti, Labanchi, Herrero, Cantoni, Perez and Odriozola2004). In agreement with that report, 52.6% of the patients in our study had no symptoms before the disease was diagnosed. Hepatic CE usually causes pain in the upper abdominal region, hepatomegaly, cholestasis, biliary cirrhosis, portal hypertension and a variety of other manifestations (Ammann & Eckert, Reference Ammann and Eckert1996). The most frequently reported symptoms in our patients, non-specific right upper abdominal pain and nausea, were observed in 47.4% of cases, which correlates well with the findings of other studies (Eckert & Deplazes, Reference Eckert and Deplazes2004; Dopchiz et al., Reference Dopchiz, Elissondo, Andresiuk, Maiorini, Gutierrez, Muzulin, Rosenzvit, Lavallen and Denegri2009). In our study, size of the cyst did not correlate with the presence of symptoms. The average cyst size in symptomatic patients was 5.1 ± 1.5 cm, whereas the mean size in asymptomatic patients was 4.5 ± 2.2 cm. The frequency of unique cysts (78.9%) detected in this study is in agreement with the findings of other studies (Moro & Schantz, Reference Moro and Schantz2009; Khanfar, Reference Khanfar2014). Larrieu & Frider (Reference Larrieu and Frider2001) reported that in symptomatic patients cysts measured 5.79–15.03 cm.
The treatment of CE has changed in recent years with the development of benzimidazole compounds; albendazole is generally the drug of choice. Chemotherapy with albendazole is apparently more effective in young patients than in older patients, and thin-walled, small cysts are the most susceptible to the treatment (Kern, Reference Kern2006). As the drug of choice, albendazole has been used pre- and post-operatively, for days or months; however, its efficacy and usefulness are not entirely clear (Brunetti et al., Reference Brunetti, Kern and Vuitton2010). Certain factors influence the results of therapeutic treatment: the drug used, the age of the cyst and the age of the patient, morphology and localization of the cyst, differences in strains of E. granulosus and intrinsic sensitivity of the cyst (Stamatakos et al., Reference Stamatakos, Sargedi, Stefanaki, Safioleas, Matthaiopolou and Safioleas2009). Kern (Reference Kern2006) emphasized the importance of the duration of therapy as a factor in the outcome of albendazole treatment. Patients who received albendazole therapy for 1 or 3 months before surgery had cyst viability of 28% and 6%, respectively. Regarding treatment of the disease, the same study showed that surgery was the therapeutic choice in all patients. In our study, 31.6% (12/38) of the patients were treated pre-operatively with albendazole for 1 month at a dosage of 10 mg/kg per day. We found that in 91.6% of the patients, the viability test was still positive after surgery. Among the patients who did not receive albendazole treatment, 73% had viable cysts. This finding leads us to conclude that albendazole has limited efficacy when administered pre-operatively.
Serological tests, especially ELISA, are useful for confirming presumptive imaging diagnoses (Ortona et al., Reference Ortona, Riggano, Buttari, Delunardo, Ioppolo, Margutti, Profumo, Teggi, Vaccari and Siracusano2003; Kilimcioglu et al., Reference Kilimcioglu, Girginkardesler, Korkmaz, Ozkol, Duzgun, Ostan, Pabuscu, Dinc and Ok2013). The results of serological tests depend on many factors, such as antigen quality, the test system, individual variability of immune responses, organ site and the number of hydatid cysts. However, the limitations of serodiagnosis in CE must be understood to correctly interpret the findings (Schantz, Reference Schantz2006). The parasitic proteins that have major immunodiagnostic value in detecting anti-E. granulosus antibodies are antigen 5 and antigen B (Ortona et al., Reference Ortona, Riggano, Buttari, Delunardo, Ioppolo, Margutti, Profumo, Teggi, Vaccari and Siracusano2003). The antigen B ELISA test was used previously in two studies conducted in Serbia. The first study involved 212 patients from different parts of Serbia who underwent ultrasonography for different abdominal problems, and in whom liver CE was considered in the differential diagnosis. Seropositivity was proven in 12.26% of the patients (Radonjic et al., Reference Radonjic, Džamić, Arsić Arsenijević, Đukić and Mitrović2007). The second study analysed 30 patients after liver CE was surgically removed, demonstrating ELISA seropositive results in 63% of the patients; the remaining 37% were seronegative (Culafic et al., Reference Culafic, Katić-Radivojević, Kerkez, Vukčević, Ranković and Stefanović2007).
In the present study, of the 38 patients with post-operatively confirmed liver CE, 29% were seronegative. Similar to our results, Shambesh et al. (Reference Shambesh, Craig, Wen, Rogan and Palillo1997) found that 15% of patients with a surgically verified diagnosis of liver CE were seronegative, along with 23% seronegative asymptomatic carriers. Orduna et al. (Reference Orduna, Zarzosa, Abad, Bratos, Sainz, Gutierrez, Lorenzo and Rodgriguez Torres1997) demonstrated seronegativity in 20% of surgically treated patients with an ELISA for antigen B. The reasons for seronegativity found in some patients with CE remain unclear. Some investigations offer a low antibody titre in asymptomatic patients, in patients in the early stage of the disease and in patients with calcified cysts or cysts without protoscolices and hooks, as possible reasons for seronegative findings in a subset of patients (Ortona et al., Reference Ortona, Riggano, Buttari, Delunardo, Ioppolo, Margutti, Profumo, Teggi, Vaccari and Siracusano2003).
In conclusion, liver CE should be considered in patients with exposure risk, such as those who have travelled to regions of high prevalence such as the Balkan countries. Although Serbia is an endemic region for CE, rigorous epidemiological studies encompassing definitive and intermediate hosts have not been conducted for the past three decades. The increasing number of stray dogs shedding E. granulosus eggs in urban public areas might be the reason for the significant difference in the rate of infection between urban and rural areas in the present study. In addition, uncontrolled slaughtering of livestock, common in rural settlements, and feeding the infected viscera to dogs favour the maintenance of the parasite cycle in our country. The significant progress in CE diagnosis in the past decade, as well as effective education and development of preventive and therapeutic strategies, will surely result in a reduced number of new cases. We believe that the results of our study will encourage physicians, veterinarians and health authorities to initiate a programme to prevent and control CE in the Balkan region.
Acknowledgements
The authors would like to thank Professor Renaud Piarroux from the Parasitology Department of the University Hospital Timone, Marseille, France, for useful advice and assistance, and all the staff working in the Department of Parasitology–Mycology, Institute of Microbiology and Immunology, University of Belgrade Faculty of Medicine.
Financial support
This work was supported partially by the Ministry of Education, Science and Technological Development, Republic of Serbia (grant no. III45005).
Conflict of interest
None.
Ethical standards
The study was approved by the Ethics Committee of the Faculty of Medicine, University of Belgrade, Serbia. The patients consented voluntarily to participate in the study, maintaining their anonymity.







