Introduction
Ovine parasitic infections by gastrointestinal helminths have an enormous economic impact in terms of morbidity, mortality, decreased production of milk, meat and wool (Chartier & Hoste, Reference Chartier and Hoste1994; Akhtar et al., Reference Akhtar, Iqbal, Khan and Lateef2000; Githiori et al., Reference Githiori, Athanasiadou and Thamsborg2006). Among the dominant species of nematodes of sheep, Haemonchus contortus causes severe damage to animal health because of its high prevalence and its pathogenicity, related to its haematophagous habits (Angulo-Cubillán et al., Reference Angulo-Cubillán, García-Coiradas and Cuquerella2010; Hoberg et al., Reference Hoberg, Kumsa, Pilitt and Abrams2010; Besier et al., Reference Besier, Kahn, Sargison and Van Wyk2016a). This blood-feeding activity is the main sign of haemonchosis, leading to anaemia, weakness and, frequently, to death (Besier et al., Reference Besier, Kahn, Sargison and Van Wyk2016b). In Tunisia, the prevalence of H. contortus in sheep and goats has recently been estimated at 17.0% and 33.6%, respectively (Akkari et al., Reference Akkari, Jebali, Gharbi, Mhadbi, Awadi and Darghouth2013).
The most commonly used method to control these gastrointestinal parasitic nematodes is based on the repeated use of chemical agents (Knox et al., Reference Knox, Besier, Le Jambre, Kaplan, Torres-Acosta, Miller and Sutherland2012; Leathwick, Reference Leathwick2012). However, the repeated use of anthelmintics has several disadvantages and limitations, including high toxicity, high cost, the presence of residues in the environment and in consumer products, and the increasing resistance of parasites to drugs (Chartier & Hoste, Reference Chartier and Hoste1994; Waller, Reference Waller2006; Peña-Espinoza et al., Reference Peña-Espinoza, Thamsborg, Demeler and Enemark2014; Van den Brom et al., Reference Van den Brom, Moll, Kappert and Vellema2015). In this context, searching for promising approaches to identify new natural candidates for parasitic control in sheep is important (Alonso-Díaz et al., Reference Alonso-Díaz, Torres-Acosta, Sandoval-Castro and Hoste2010). In this regard, the present study focused on polyphenol-rich plants as a natural alternative to the classical anthelmintics.
Polyphenols have been considered to be the main active compounds responsible for anti-oxidant and bio-functional properties detected in many medicinal plants. Many in vitro and in vivo experiments have confirmed their protective activity against a multitude of human and animal conditions, such as allergy, inflammation and viral infections (Cutler et al., Reference Cutler, Nettleton, Ross, Harnack, Jacobs, Scrafford, Barraj, Mink and Robien2008; Carocho & Ferreira, Reference Carocho and Ferreira2013). Phenolic compounds have been proposed to treat abomasal and intestinal parasitic nematodes (Hoste et al., Reference Hoste, Jackson, Athanasiadou, Thamsborg and Hoskin2006). Moreover, a relationship between polyphenols, flavonoids and tannins and the anthelmintic activity against H. contortus has been reported in a previous study (Akkari et al., Reference Akkari, Hajaji, B'chir, Rekik and Gharbi2016).
Chamomile (Matricaria recutita L.), an annual herbaceous plant of the Asteraceae family, is one of the most widely used and well-documented medicinal plants in the world (Gupta et al., Reference Gupta, Mittal, Bansal, Khokra and Kaushik2010) and is generally regarded as safe (GRAS) because it neither contains toxic compounds nor represents any acute toxicity for humans and animals (Bradley, Reference Bradley1993; Tolouee et al., Reference Tolouee, Alinezhad, Saberi, Eslamifar, Zad, Jaimand, Taeb, Rezaee, Kawachi, Shams-Ghahfarokhi and Razzaghi-Abyaneh2010). It has been used for centuries as a medicinal plant, mostly for a variety of illnesses related to inflammation of the skin, upper respiratory and gastrointestinal tract (Petronilho et al., Reference Petronilho, Maraschin, Coimbra and Rocha2012). It has several benefits for human and animal health such as antimicrobial, antidiarrhoeal, anti-oxidant, antispasmodic, anti-inflammatory, anti-allergic and antidepressant properties (McKay & Blumberg, Reference McKay and Blumberg2006; Cemek et al., Reference Cemek, Yilmaz and Büyükokuroğlu2010; Chandrashekhar et al., Reference Chandrashekhar, Halagali, Nidavani, Shalavadi, Biradar, Biswas and Muchchandi2011; Al Bahtiti, Reference Al Bahtiti2012; Amsterdam et al., Reference Amsterdam, Shults, Soeller, Mao, Rockwell and Newberg2012; Sebai et al., Reference Sebai, Jabri, Souli, Rtibi., Selmi, Tebourbi, El-Benna and Sakly2014). Chamomile is known for its richness in phenolic compounds believed to be responsible for its biological activities (Gardiner, Reference Gardiner2007; Guimarães et al., Reference Guimarães, Barros, Dueñas, Calhelha, Carvalho, Santos-Buelga, Queiroz and Ferreira2013). It contains phenolic compounds such as the flavonoids apigenin, quercetin, patuletin and luteolin, glucosides and coumarins (herniarin and umbelliferone) (Mann & Staba, Reference Mann, Staba, Craker and Simon1986; McKay & Blumberg, Reference McKay and Blumberg2006).
As far as could be ascertained, there is no published report in the literature on the anthelmintic activity of chamomile. The present study aimed to investigate the radical-scavenging activity of M. recutita L. flower extracts obtained using solvents of increasing polarity. Flower extracts were also tested for their anthelmintic effects against H. contortus from sheep. These activities were correlated with the phenolic content of the plant extracts.
Materials and methods
Plant material and preparation of extracts
Chamomile flowers were cultivated from the region of Beja (north-west of Tunisia, altitude 448 m; 36°81′N; 9°05′E) during spring 2013 and identified by Mouhiba Ben-Naceur, the Professor of Taxonomy in the Higher Institute of Biotechnology of Beja-Tunisia. Chamomile flowers were separated and thoroughly rinsed in running tap water then air dried for 14 days. The flowers were then ground to a fine powder in an electric blender (Moulinex Ovatio 2, Serris, France) and finally stored at 4°C until extraction.
Extracts were prepared by dissolving 100 g of powdered plant material in 500 ml of extraction solvent with increasing polarity (water, methanol, chloroform and hexane). After 24 h of maceration at room temperature (20–25°C, 3 × 500 ml) in the dark, to prevent light degradation, tested extracts were collected and filtered three times using Whatman No. 1 paper, then a rotary vacuum evaporator at 40°C was used in order to remove the solvent, and the aqueous filtrate was lyophilized using a lyophilizer.
Plant extract analysis
Concentrations of total polyphenols, flavonoids and tannins in flower extracts of M. recutita were determined by colorimetric methods.
Total phenolic content
Total phenolic content was determined by colorimetry using the Folin–Ciocalteu reagent (FCR) according to the method described by Singleton et al. (Reference Singleton, Orthofe and Lamuela-Ravento1999). An aliquot of 100 μl of each tested extract was added to 500 μl of FCR previously diluted in water (1/10). A total volume of 4 ml of Na2CO3 solution was then added to the mixture and mixed thoroughly before incubation for 15 min at room temperature. The absorbance versus a prepared blank was measured at 765 nm. Total phenolic content was expressed as mg gallic acid equivalents/g dry weight (mg of GAE/g DW) using a calibration curve with gallic acid. The calibration curve range was 50–200 mg/ml. Triplicate measurements were performed for all samples.
Total flavonoid content
Total flavonoid content was measured by the colorimetric method using aluminium ion Al3+ (Bahorun et al., Reference Bahorun, Gressier, Trotin, Brunet, Dine, Luyckx, Vasseur, Cazin, Cazin and Pinkas1996). To each extracted sample, 1.5 ml of AlCl3 (2%) solution were added. The mixture was then shaken thoroughly before incubation for 10 min at room temperature. The absorbance versus a prepared blank was measured at 367 nm. Total flavonoid content was expressed as mg quercetin equivalents/g dry weight (mg QE/g DW) through a previously established quercetin calibration curve. Concentrations ranged from 15 to 100 g/ml. Triplicate measurements were performed for all samples.
Condensed tannin content
In the presence of concentrated HCl, condensed tannins were transformed by the reaction with vanillin to anthocyanins (Sun et al., Reference Sun, Leandro, Ricardo-da-Silva and Spranger1998). Fifty microlitres of each sample extract, appropriately diluted, were mixed with 3 ml of 4% methanol vanillin solution and 1.5 ml of HCl. After 15 min, the absorbance was measured at 500 nm. Condensed tannin contents were expressed as mg catechin equivalents/g dry weight (mg CE/g DW) through a calibration curve with catechin. The calibration curve range was 50–600 mg/ml.
Anti-oxidant activity evaluation
The anti-oxidant activity of each tested extract was measured using free-radical scavenging activity by DPPH (2,2-diphenyl-1-picrylhydrazyl) and by ABTS (2,2-azino-bis[3-ethylbenzthiazoline-6-sulphonic acid]) radical cation.
Free-radical scavenging ability using a stable DPPH radical
The free-radical scavenging activity of extracts was measured by DPPH using the method described by Gorinstein et al. (Reference Gorinstein, Haruenkit, Park, Jung, Zachwieja, Jastrzebski, Katrich, Trakhtenberg and Belloso2004). A volume of 2.8 ml solution of DPPH in methanol (0.1 mm) was added to 0.2 ml of each extract sample in methanol at different concentrations (1.56, 3.12, 6.25, 12.50, 25.00, 50.00 and 100.00 μg/ml), incubated in a dark room for 30 min, then the absorbance was measured at 517 nm. Radical scavenging activity was calculated using the following equation: Scavenging effect = [(A 0 − A 1)/A 0] × 100, where A 0 is the absorbance of the control and A 1 is the absorbance in the presence of the sample. The extract concentration providing 50% inhibition (IC50) was calculated from the plot of inhibition percentage against extract concentration. Tests were carried out in triplicate.
Free-radical scavenging ability using a stable ABTS radical cation
The free-radical scavenging activity was determined by the ABTS radical cation decolourization assay described by Siddhuraju (Reference Siddhuraju2006). ABTS was dissolved in water to a 7 mm concentration. ABTS radical cation (ABTS+) was produced by reacting ABTS stock solution with 2.45 mm potassium persulphate and was kept in a dark room at room temperature for 12–16 h before use. The ABTS+ was diluted to the absorbance of 0.70 ± 0.02 and stocked for off-line and on-line assays. A volume of 1 ml of each diluted tested extract at different concentrations (1.56, 3.12, 6.25, 12.50, 25.00, 50.00 and 100.00 g/ml) was added to 3 ml of ABTS+ solution and kept in a dark place at room temperature for 60 min. The absorbance was measured at 734 nm. The scavenging capacity was calculated as [(A 0 – Ab)/A 0] × 100, where Ab is the absorbance in the presence of the sample, and A 0 refers to the absorbance of ABTS+ without sample. The extract concentration providing 50% inhibition (IC50) was calculated from the plot of inhibition percentage against chamomile extract concentration. All tests were performed in triplicate. For both DPPH and ABTS assays, the same procedure was repeated with a synthetic anti-oxidant – ascorbic acid – as positive control. Anti-oxidative capacities of the extracts were compared with those of ascorbic acid.
In vitro anthelmintic assays
The anthelmintic efficacy tests of chamomile extracts on H. contortus were performed using two different procedures. For each assay, the eggs or adult stages were obtained from faeces and abomasum of Barbarine donor lambs experimentally infected by oral administration of a pure aqueous suspension of 6000 H. contortus third-stage larvae (L3), according to the World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines (Coles et al., Reference Coles, Bauer, Borgsteede, Greerts, Klei, Taylor and Waller1992).
Egg-hatch assay
Freshly collected eggs were incubated with different extracts in quadruplet. Dried flower extracts of M. recutita were used as a test treatment. Untreated eggs in phosphate-buffered saline (PBS) with dimethyl sulphoxide (DMSO) (0.5%) solution were used as a negative control. Albendazole reference drug (99.8% pure standard reference, Médivet, SA, Tunisia) was dissolved in DMSO and diluted at three concentrations (0.25, 0.50 and 1.00 μg/ml); eggs treated with the different concentrations of albendazole served as positive controls. For each extract concentration, approximately 200 eggs in 1 ml of PBS were placed in each test tube. Sample extracts at different concentrations (8.0, 4.0, 2.0, 1.0, 0.5 and 0.25 mg/ml) in 1 ml PBS with DMSO (0.5%) were used. After incubation for 48 h at 27°C, egg hatching was stopped by adding Lugol's iodine solution. The numbers of L1 larvae and eggs per well were counted under a dissecting microscope at 40× magnification. The percentage of hatched eggs was determined using the ratio: number of L1 × 100/(number of eggs + number of L1).
Adult worm motility assay
This test was performed according to Hounzangbe-Adote et al. (Reference Hounzangbe-Adote, Paolni, Fouraste, Moutairou and Hoste2005). Adult worms were collected from a lamb, at week 6 after experimental infection. Immediately after being slaughtered, the abomasum was removed, opened and placed at 37°C in 0.9% sodium chloride solution. The collected parasites were then washed and kept in PBS solution. Eight actively moving worms were placed in Petri dishes filled with 8.0, 4.0, 2.0 and 1.0 mg/ml of M. recutita extracts in PBS with DMSO (0.5%), in a volume of 3 ml. Albendazole dissolved in DMSO then in PBS at a final concentration of 1 mg/ml was used as a positive control. PBS with DMSO (0.5%) was used as a negative control. All the tests were performed in triplicate. Inhibition of worm motility was the criterion of anthelmintic activity. The required delays for worm paralysis and/or complete immobility were recorded at 0, 1, 2, 4, 6 and 8 h. To test if the worms could recover their motility after incubation for 8 h, they were washed with distilled water and resuspended in PBS for 30 min. Worm death was ascertained by the absence of motility during an observation period of 5–6 s. The immobility index was calculated as follows: Immobility index (%) = 100 × (number of dead worms per Petri dish/total number of worms per Petri dish).
Statistical analysis
The statistical analysis was performed using the SPSS 10.0 software package for Windows (SPSS Inc., Chicago, Illinois, USA). SigmaPlot 12.0 (Systat Software Inc., San José, California, USA) was used to calculate the IC50 for ABTS, DPPH and egg-hatch inhibition.
Regression was used for evaluation of the dose–response relationship using Minitab® Release 14 (Minitab SARL (France) www.minitab.com). The result of the worm motility inhibition was expressed as mean ± standard error of mean (SEM). The results of IC50 for egg-hatching inhibition were assessed statistically using analysis of variance (ANOVA) and complemented by multiple comparisons of means by the SNK test (Student–Newman–Keuls). Spearman's correlation coefficients were calculated between anti-oxidant capacity and anthelmintic effects versus polyphenolic content, between DDPH and ABTS assays, DDPH and egg-hatching assays and, finally, between ABTS and egg-hatching assays of tested extracts (SPPS 10.0 software). Means of DDPH, ABTS scavenging activities and anthelmintic efficacy were compared by Wilcoxon test (SPSS 10.0 software). A probability of 0.05 was used as the threshold for statistical significance.
Results
Extract yields
The highest percentage yield of extraction of M. recutita was in the aqueous extract (11.18%) while yields of the methanolic, chloroformic and hexanic extracts were 10.54, 9.12 and 8.22%, respectively (table 1).
Table 1. Extract yields, polyphenolic content and effect of Matricaria recutita extracts on free-radical scavenging capacity and egg-hatching activity.

TP, Total phenolic content expressed as mg gallic acid equivalents/g dry weight (mg GAE/g DW); TF, total flavonoid content expressed as mg quercetin equivalents/g dry weight (mg QE/g DW); TC, condensed tannin contents expressed as mg catechin equivalents/g dry weight (mg CE/g DW); DDPH, 2,2-diphenyl-1-picrylhydrazyl; ABTS, 2,2-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid); IC50, value of concentration causing 50% inhibition of the activity.
Total phenolic, flavonoid and condensed tannin contents
Phytochemical screening revealed the presence of polyphenols, flavonoids and condensed tannins in all the tested extracts. Their amounts varied according to the nature of the extract.The highest concentration was found in the methanolic extract (42.12 ± 2.30, 28.45 ± 0.19 and 7.42 ± 0.33 mg/g DW, respectively), while the chamomile hexanic extract gave the lowest amount of these compounds (1.67 ± 0.20, 4.64 ± 0.29 and 0.37 ± 0.04 mg/g DW, respectively) (table 1).
Anti-oxidant activity
The radical scavenging activity of chamomile extracts and ascorbic acid increased significantly in a dose-dependent manner (fig. 1). Inhibitory concentrations for methanol and aqueous extract (IC50) were 1.19 and 1.38 μg/ml, respectively, for ABTS and 1.18 and 1.23 μg/ml, respectively, for DPPH scavenging activities. These two extracts showed important anti-oxidant activity, being close to ascorbic acid (IC50 = 1.16 and 1.17 μg/ml for DPPH and ABTS scavenging activities, respectively) (table 1, fig. 1).
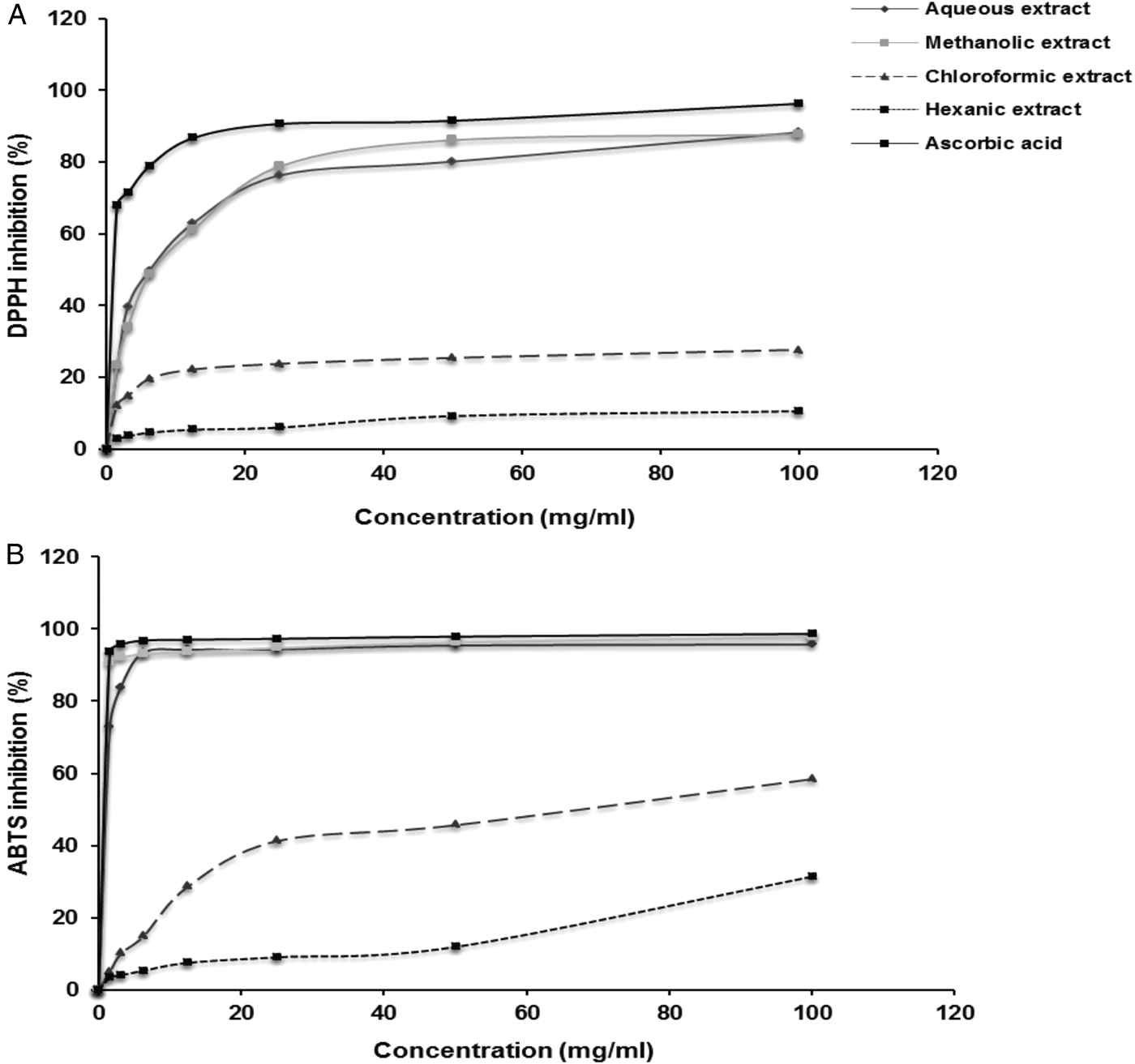
Fig. 1. Free-radical scavenging activities of aqueous, methanolic, chloroformic and hexanic extracts of Matricaria recutita and ascorbic acid measured by (A) DPPH and (B) ABTS methods.
The comparison between DPPH and ABTS scavenging activities showed that there was a statistically significant correlation with aqueous, methanolic, chloroformic and hexanic extracts (r = 0.915, 0.758, 0.948 and 0.915, respectively; P < 0.01) (table 2). Also, a high positive correlation was observed between total polyphenols, flavonoids and condensed tannins with scavenging of DPPH (0.764, 0.722 and 0.731, respectively; P < 0.01) and ABTS radicals (0.722, 0.752 (P < 0.01) and 0.604 (P < 0.05), respectively) (table 3).
Table 2. Pearson's correlation coefficient between DDPH and ABTS assays, DDPH and egg-hatching assays, and ABTS and egg-hatching assays of tested extracts of Matricaria recutita.

**, P < 0.01.
Table 3. Correlation coefficients of total phenolic, total flavonoid and condensed tannin contents versus DPPH, ABTS radical scavenging activity and egg-hatching inhibition of M. recutita extracts.

**, P < 0.01; *, P < 0.05.
In vitro anthelmintic assays
Egg-hatch assay
Methanolic and aqueous extracts from chamomile showed higher inhibitory effects on egg hatching (IC50 = 1.523, 2.559 mg/ml, respectively) (table 1). The inhibitory effect of the hexanic extract was the lowest, as it induced 42.06% egg-hatching inhibition at 8 mg/ml (fig. 2). Albendazole inhibited 91.75% of egg hatching at 1 μg/ml (IC50 = 0.1255 μg/ml). The maximum concentration required to induce total (100%) egg-hatch inhibition for the methanolic extract was 4 mg/ml (table 1).

Fig. 2. Dose-dependent profile of the egg-hatching inhibition of Haemonchus contortus submitted to increasing concentrations of plant extract (0, 0.25, 0.5, 1, 2, 4, 8 mg/ml). Standard errors of the mean are shown on each bar. Values labelled with superscript letters (a–d) are significantly different (P < 0.001), n = 4.
On the one hand, the results showed a high correlation between the inhibition of egg hatching and total polyphenols, flavonoids and tannins (r = 0.747, 0.749 and 0.818, respectively, P < 0.01) (table 3). On the other hand, the inhibition of egg hatching was significantly correlated with scavenging of both DPPH (r > 0.640; P < 0.01) and ABTS radicals (r > 0.747; P < 0.01) in all tested extracts (table 2).
Adult worm motility assay
All extracts produced in vitro showed dose-dependent activity on adult parasites. The methanolic extract, however, killed more worms than the other extracts at all concentrations tested (P < 0.05). After 8 h, it induced 91.77% mortality at the highest concentration tested (8 mg/ml), while the aqueous extract induced only 75.05% mortality at the same concentration (table 4). There was 82.26% mortality of worms in albendazole within 8 h post-exposure. However, the worms in the DMSO negative control solution showed neither paralysis nor mortality. Finally, no worm recovered motility in the PBS revival test.
Table 4. In vitro anthelmintic efficacy of extracts of Matricaria recutita on Haemonchus contortus.

a Albendazole is a positive control; PBS with DMSO 0.5% is a negative control.
C, concentration.
Discussion
In this study, M. recutita flower extracts recovered using solvents of increasing polarity were examined for their anti-oxidant and anthelmintic activities.
First, the results of the phytochemical study showed that M. recutita was rich in polyphenols, flavonoids and condensed tannins. Their quantities were higher in methanolic and aqueous extracts compared to extracts in other organic solvents (chloroform and hexane). The phenolic contents of the tested extracts were higher than that of the decoction extract from Tunisian M. recutita (Sebai et al., Reference Sebai, Jabri, Souli, Hosni, Rtibi, Tebourbi, El-Benna and Sakly2015) and they were higher than those of the extracts of Brazilian chamomile tea and infusions (Guimarães et al., Reference Guimarães, Barros, Dueñas, Calhelha, Carvalho, Santos-Buelga, Queiroz and Ferreira2013; Zielinski et al., Reference Zielinski, Haminiuk, Alberti, Nogueira, Demiate and Granato2014). However, many other studies showed that ethanolic and aqueous extracts contain more phenolic compounds than the extracts tested in this study (Vinha et al., Reference Vinha, Soares, Castro, Santos, Oliveira and Machado2012; Alibabaei et al., Reference Alibabaei, Rabiei, Rahnama, Mokhtari and Rafieian-kopaei2014). This could be attributed to the geographic origin and the variety of chamomile (Sharafzadeh & Alizadeh, Reference Sharafzadeh and Alizadeh2011) as well as the climatic conditions and the extraction process (Papagiannopoulos et al., Reference Papagiannopoulos, Wollseifen, Mellenthin, Haber and Galensa2004; Sebai et al., Reference Sebai, Jabri, Souli, Hosni, Rtibi, Tebourbi, El-Benna and Sakly2015). Moreover, the extracting solvent has a prominent impact on the amount and nature of the compounds recovered, so the difference in phytochemical profile can modify the efficacy and the biological activity of the extract (Makris & Kefalas, Reference Makris and Kefalas2004; Naczk & Shahidi, Reference Naczk and Shahidi2006; Sifaoui et al., Reference Sifaoui, Lopez-Arencibia, Martin-Navarro, Chammem, Reyes-Batlle, Mejri, Lorenzo-Morales, Abderabba and Pinero2014).
The results of this study are consistent with previous results from other studies, which confirmed the high concentration of phenolic compounds in chamomile (Guimarães et al., Reference Guimarães, Barros, Dueñas, Calhelha, Carvalho, Santos-Buelga, Queiroz and Ferreira2013; Mekinić et al., Reference Mekinić, Skroza, Ljubenkov, Krstulović, Možina and Katalinić2014). Many authors showed that polar solvents, especially methanol and ethanol, are more efficient in extracting these phenolic compounds because they have high polarity and good solubility (Roby et al., Reference Roby, Sarhan, Selim and Khalel2013; Formisano et al., Reference Formisano, Delfine, Oliviero, Tenore, Rigano and Senatore2015).
Beside the phytochemical composition, free-radical scavenging activity of different extracts was investigated by two methods: DPPH and ABTS. Results showed that all chamomile extracts were characterized by important anti-oxidant activities for both tests. Methanolic and aqueous extracts showed the best scavenging activity, with lower values compared to chloroformic and hexanic extracts as well as other plant extracts well known for their anti-oxidant properties (Kelebek et al., Reference Kelebek, Selli, Canbas and Cabaroglu2009; Zhao et al., Reference Zhao, Wang, Cheng, Yao, Wang, Lu, Yang and Ma2011).
For all extracts, a significant and positive correlation between DPPH and ABTS tests was observed. Therefore, the current results highlight that total phenolic, total flavonoid and condensed tannin contents were positively correlated with both ABTS and DPPH radical assays. This means that the good anti-oxidant capacity of chamomile is mainly related to the high levels of polyphenols and flavonoids. This correlation was supported by many authors and is common among the majority of plants (Pyo et al., Reference Pyo, Lee, Logendra and Rosen2004; Hamad et al., Reference Hamad, Erol-Dayi, Pekmez, Önay-Uçar and Arda2010; Guimarães et al., Reference Guimarães, Barros, Dueñas, Calhelha, Carvalho, Santos-Buelga, Queiroz and Ferreira2013; Akkari et al., Reference Akkari, Hajaji, B'chir, Rekik and Gharbi2016). The findings of this study corroborate previous reports suggesting that phenols and flavonoids contained in chamomile methanolic extract strongly contribute to the anti-oxidant activity (Formisano et al., Reference Formisano, Delfine, Oliviero, Tenore, Rigano and Senatore2015). Recent findings suggest that especially quercetin (flavonol), chlorogenic acid, caffeic acid, p-coumaric acid and ferulic acid (phenolic acids) are the primary sources of anti-oxidant ability of chamomile methanolic extract, by scavenging free radicals such as the hydroxyl radical (OH), which is the major cause of lipid peroxidation (Nováková et al., Reference Nováková, Vildová, Mateus, Gonçalves and Solich2010; Roby et al., Reference Roby, Sarhan, Selim and Khalel2013).
Our results confirm the anthelmintic effect of chamomile extracts on H. contortus. Methanolic extract totally inhibited egg hatching at low concentration (4 mg/ml) compared to aqueous, chloroformic and hexanic extracts. These results confirm those of a previous study, in which the methanolic extract from Rubus ulmifolius inhibited 95.66% of egg hatching at 4 mg/ml (Akkari et al., Reference Akkari, Hajaji, B'chir, Rekik and Gharbi2016). However, ethanolic extracts of Artemisia campestris and Thymys capitatus had good activity at a lower concentration (2 mg/ml) (Boubaker Elandolsi et al., Reference Boubaker Elandolsi, Akkari, Bchir, Gharbi, Mhadbi, Awedi and Darghouth2013; Akkari et al., Reference Akkari, Rtibi, B'chir, Rekik, Darghouth and Gharbi2014).
The findings of the current study also provide evidence that chamomile extracts have significant impact on adult H. contortus, observed in terms of worm paralysis and/or death at different post-treatment intervals at even low concentrations. Previous studies revealed that R. ulmifolius exhibited significant activity at the same concentrations (Akkari et al., Reference Akkari, Hajaji, B'chir, Rekik and Gharbi2016); meanwhile, A. campestris and T. capitatus were active at lower concentrations (100% inhibition at 2 mg/ml) (Boubaker Elandolsi et al., 2013; Akkari et al., Reference Akkari, Rtibi, B'chir, Rekik, Darghouth and Gharbi2014).
Based on these results, a significant linear positive correlation between total polyphenols, flavonoids and tannin contents and anthelmintic activity was observed. Akkari et al. (Reference Akkari, Hajaji, B'chir, Rekik and Gharbi2016) reported the same result with Rubus extracts. Many hypotheses have been proposed to explain the antiparasitic activity of these compounds, which are known to interfere with energy generation in parasites by uncoupling oxidative phosphorylation (Athnasiadou et al., Reference Athanasiadou, Kyriazakis, Jackson and Coop2001).
Tannins are known for their protein-binding ability, which can protect proteins from degradation in the rumen, and increase protein flow to the small intestine and amino acid absorption (Min et al., Reference Min, Barry, Attwood and McNabb2003; Waghorn & McNabb, Reference Waghorn and McNabb2003; Hoste et al., Reference Hoste, Jackson, Athanasiadou, Thamsborg and Hoskin2006). Their antiparasitic activity on adults can be explained by their contact with the nematode's cuticle, buccal cavity, oesophagus and reproductive tract (Hoste et al., Reference Hoste, Martínez-Ortiz-De-Montellano, Manolaraki, Brunet, Ojeda-Robertos, Fourquaux, Torres-Acosta and Sandoval-Castro2012; Martínez-Ortíz-de-Montellano et al., Reference Martínez-Ortíz-de-Montellano, Arroyo-López, Fourquaux, Torres-Acosta, Sandoval-Castro and Hoste2013). Moreover, polyphenolic compounds might interfere with enzymes secreted or excreted by the worms in the local environment, or with enzymes involved in metabolic pathways that are essential for nematode functions (Athanasiadou et al., Reference Athanasiadou, Kyriazakis, Jackson and Coop2001).
The study of structural changes in adult H. contortus after in vitro exposure to medicinal plants has been the focus of several authors. Martínez-Ortíz-de-Montellano et al. (Reference Martínez-Ortíz-de-Montellano, Arroyo-López, Fourquaux, Torres-Acosta, Sandoval-Castro and Hoste2013) studied two plants rich in tannins – sainfoin (Onobrychis viciifolia) and tzalam (Lysiloma latisiliquum) – and showed that their effects were observed mainly in the cuticle, the cephalic region, the vulva and the anus of female worms. Microscopic observations showed longitudinal and transverse folds and thick crests in the cuticle. These lesions were observed either on the whole body or on patches along the whole body of the nematode, including the cephalic parts and the rest of the body, as well as the distal part of the worms. Thus, aggregates around the oral capsule, female vulva or anus have been observed and are similar under both in vivo and in vitro conditions.
The strong correlation between anti-oxidant and antiparasitic activity of chamomile can be explained by its rich flavonoid content. Interestingly, it has been suggested that anti-oxidant flavonoids might have anthelmintic activity (Ferreira, Reference Ferreira2011) and anti-oxidant levels are also an indicator of flavonoids that can potentiate commercial anthelmintics. In fact, the anti-oxidant flavonoid quercetin is widely found in plants, and has been reported to increase the anthelmintic activity of moxidectin in lambs (Dupuy et al., Reference Dupuy, Larrieu, Sutra, Lespine and Alvinerie2003). Nevertheless, chamomile can be used to reduce parasitic burdens and to improve both animal production and health (Wangchuk, Reference Wangchuk2008).
Based on the results of the present study, chamomile extracts showed significant dose-dependent free-radical scavenging and in vitro anthelmintic activity against H. contortus, as ascertained by worm motility and egg-hatching inhibition. The presence of phenolic compounds, such as flavonoids and tannins, is particularly important for expressing various bioactivities. Chamomile may thus be exploited for the development of new natural anti-oxidant and anthelmintic agents. However, bio-guided purification will be necessary to isolate and purify bioactive compounds from chamomile flower extracts, to evaluate their in vivo activities and to study the structural changes in the worms by electron microscopy. This will be of great value to determine the mode of action of chamomile on the worms.
Acknowledgements
We are grateful to Mr Sassi Limam, Mr Mohamed Jedidi, Mr Bechir Guesmi and Mr Tawfik Lahmar for their valuable technical assistance.
Financial support
This work received financial support from ‘Laboratoire d'Epidémiologie d'Infections Enzootiques des Herbivores en Tunisie’ (Ministère de l'enseignement supérieur, Tunisia).
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and use of laboratory animals and have been approved by the institutional committee of the Institut Pasteur, Tunis.








