Introduction
Asymptomatic bacteriuria (ASB) is defined by the Infectious Diseases Society of America guidelines as two sequential urine samples from women without symptoms and signs of urinary tract infection (UTI), containing 105 cfu/ml or more, of the same organism. Reference Rubin, Shapiro, Andriole, Davis and Stamm1–Reference Schnarr and Smaill3 The prevalence of ASB ranges between 2% and 7%. Reference Sheiner, Mazor-Drey and Levy4 The predominant uropathogen in ASB, for both pregnant and nonpregnant women, is E. coli. Reference Rubin, Shapiro, Andriole, Davis and Stamm1–Reference Cortes-Penfield, Trautner and Jump7
During pregnancy marked anatomical and physiological changes occur, including in the urinary system. The smooth muscle relaxation in bladder myocytes and subsequent ureteral dilatation that accompany pregnancy are thought to facilitate the ascent of bacteria from the bladder to the kidney, resulting in the greater propensity for bacteria to reach the kidneys and cause pyelonephritis. 6,Reference Mazor-Dray, Levy, Schlaeffer and Sheiner8–Reference Lai, Hsu and Lan10 In addition, pregnancy is considered as a special immunological state characterized by an attenuation of the acquired immune response that may also contribute to infection susceptibility. Reference Leeper and Lutzkanin9–Reference Kalinderi, Delkos, Kalinderis, Athanasiadis and Kalogiannidis13
Consequently, if ASB is untreated, up to 30% of mothers develop acute pyelonephritis. Reference Nicolle, Gupta and Bradley2 Pyelonephritis during pregnancy is associated with significant morbidity for both the mother and the fetus. Reference Farkash, Weintraub, Sergienko, Wiznitzer, Zlotnik and Sheiner14 Recent studies showed an association between women with untreated bacteriuria and an increased risk of preterm birth, preterm premature rupture of membranes, low birth weight (LBW), increased risk of preeclampsia, low 5-min Apgar score, and even perinatal mortality. Reference Sheiner, Mazor-Drey and Levy4,Reference Mazor-Dray, Levy, Schlaeffer and Sheiner8,Reference Kalinderi, Delkos, Kalinderis, Athanasiadis and Kalogiannidis13 Therefore, proper screening and treatment of ASB are necessary to prevent complications. Reference Nicolle, Gupta and Bradley2,6,Reference Keating15 The management of ASB includes antibiotic therapy tailored to culture results and follow-up cultures to confirm sterilization of the urine.
Long-term consequences of antibiotic treatment for maternal ASB on the offspring are not systematically discussed in the medical literature. Although antibiotic treatment of ASB reduces perinatal morbidity, effects of such treatment later on in childhood should be explored. The aim of the current study was to address the possible association between maternal prenatal ASB and the risk for long-term offspring infectious morbidity requiring hospitalization.
Methods
In this population-based retrospective cohort study, all infants born between the years 1991 and 2014 at the Soroka University Medical Center (SUMC), the largest birth center in Israel, were included. SUMC is the sole tertiary hospital in the southern region of Israel (Negev), serving the entire population in this region. Thus, the study was based on nonselective population data. The research was done in accordance with the 1964 Helsinki Declaration and its subsequent modifications' ethical principles (Helsinki Declaration 1975, revision 2013). In addition, the institutional oversight board gave their approval (SUMC IRB Committee).
The exposure group was defined as offspring to mothers with ASB that was diagnosed during routine prenatal care testing. Urine cultures are performed as part of the intensive treatment offered by Israel’s national health services to all pregnant women. Reference Sheiner, Mazor-Drey and Levy4 Multiple gestations were excluded from the study due to its association with earlier termination of pregnancy and small gestational age which increase morbidity. We also excluded from the registry fetuses with congenital anomalies due to their increased morbidity and mortality rates. Perinatal mortality cases were not included for long-term analysis. Additionally, mothers with a diagnosis of UTI during pregnancy or delivery, as well as women with insufficient prenatal care were excluded from the study. The latter may have incurred undiagnosed ASB, potentially causing misclassification in the registry.
The comparison conducted was between offspring born to mothers diagnosed with ASB during pregnancy and offspring born to nonexposed mothers, based on the perinatal database. A variety of adverse perinatal characteristics were investigated including gestational age, preterm delivery (<37 weeks’ gestation), cesarean delivery (CD), LBW (defined as birth weight <2500 g), low Apgar score at 1 and 5 min (defined as Apgar score <7), small for gestational age (SGA, defined as birth weight<5th percentile for gestational age and gender), and gender. Hospitalizations due to infectious disease with offspring under the age of 18 were analyzed using diagnoses predefined by a series of International Classification of Disease (ICD)-9 codes seen in the Supplementary Table 5. If any of the following happened, the follow-up was discontinued: hospitalization due to infectious morbidity, reaching the age of 18, hospitalization culminating in death, or completion of the research duration.
The study was based on two computerized data sets: the first is the perinatal data of the obstetric and gynecologic department in SUMC, including information that was documented by obstetricians following delivery. The second, a pool of computerized children hospitalizations in SUMC (Demog-ICD-9), includes demographic data and medical diagnosis during hospitalization. The two databases were crosslinked and merged based on the patients’ ID (mother and child). All diagnoses were classified by the ICD-9.
Statistical analysis
Bivariable analysis was performed to compare background characteristics between the two study groups, as well as the dependent variables. The bivariable analysis included Chi-square tests for categorical variables, and t-tests or Mann−Whitney U tests for continuous variables according to their distribution. Cumulative incidence rates were compared using Kaplan−Meier test using the log-rank test to determine significant differences. A Cox proportional hazards model was conducted to compare infectious-related hospitalizations risk among offspring born to mothers who were diagnosed with ASB during pregnancy and offspring born to nonexposed mothers. The model adjusted for potential confounders based on the bivariable analysis, and on clinical importance of the variables. Potential confounders included maternal age, parity, hypertensive disorders of pregnancy, diabetes mellitus, ethnicity, and gestational age.
The mothers in the cohort were entered as clusters to account for dependence between siblings. The final model was chosen based on best fit and minimal −2log likelihood. All analyses were two-sided, with a power = 80% and alpha = 0.05. The analysis was performed using SPSS package 23rd ed. as well as the STATA software 12th ed.
Results
There were 212,984 singleton deliveries that met inclusion criteria. Of them, 5378 (2.5%) mothers were diagnosed with ASB during pregnancy and their offspring considered the exposed group. Maternal characteristics of the study population by exposure status are presented in Table 1. The mothers who had been exposed to ASB during pregnancy were younger, and primigravidae. Bedouin women were more likely to have ASB as compared to Jewish women. Women with diabetes mellitus (pregestational or gestational) and hypertension (chronic, gestational, or preeclampsia) were more prone to develop ASB. Our cohort includes two different socioeconomic groups based on ethnicity – Bedouin and Jewish. According to data from government sources, rates of unemployment and low income prevail in the Bedouin society. 16
Table 1. Maternal characteristics of the study population by exposure status

a Including pregestational, gestational hypertension, and preeclampsia.
b Including pregestational and gestational diabetes.
Table 2 demonstrates the characteristics of labor and pregnancy by contrasting pregnancy outcomes between the two groups. Mean birth weight, gestational age, and Apgar score at 5 min were lower in the ASB-exposed group. Rates of preterm delivery, CD, LBW infants, SGA, and Low Apgar score at 1 min were higher in the exposed group. Table 3 displays the hospitalization rates as well as the offspring’s long-term infectious morbidities. The incidence of otorhinolaryngological, respiratory infections, skin infections, and systemic febrile syndromes rates were significantly higher in children from the ASB-exposed group. The rates of all other infectious morbidities were similar in both categories. In the group of children born to ASB-exposed mothers, the overall infectious-related hospitalization rate was significantly higher (13.1% vs. 11.1%, OR = 1.20, 95% CI 1.11–1.30, P < 0.001). Children born to mothers who had ASB during their pregnancy had a higher cumulative rate of infectious-related hospitalizations than children born to non-ASB infected mothers, according to the Kaplan–Meier survival curve (Fig. 1 log rank P = 0.006). Table 4 illustrates the association between maternal ASB during pregnancy and the long-term probability of infectious-related hospitalizations in children (up to the age of 18 years) using the Cox regression. As is being shown in Table 4, maternal ASB during pregnancy was a significant and independent risk factor for offspring’s long-term infectious-related hospitalization with an adjusted hazard ratio of 1.08 (95% CI 1.01–1.17, P = 0.042).

Fig. 1. Kaplan−Meier curve demonstrating the cumulative incidence of hospitalizations involving infectious morbidity in the offspring of exposed and nonexposed groups (log rank, P = 0.006).
Table 2. Pregnancy and delivery characteristics by exposure status
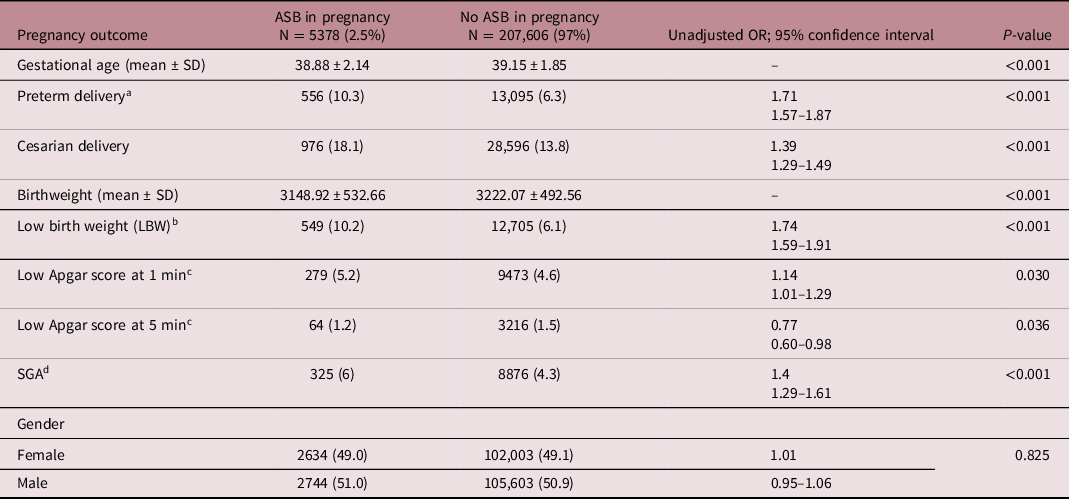
a Preterm delivery < 37+0 weeks.
b LBW < 2500 gr.
c Apgar score <7.
d BW < 10th percentile for gestational age.
Table 3. Long-term infectious morbidities requiring hospitalizations in children (up to the age of 18 years) born to mothers with and without ASB in pregnancy
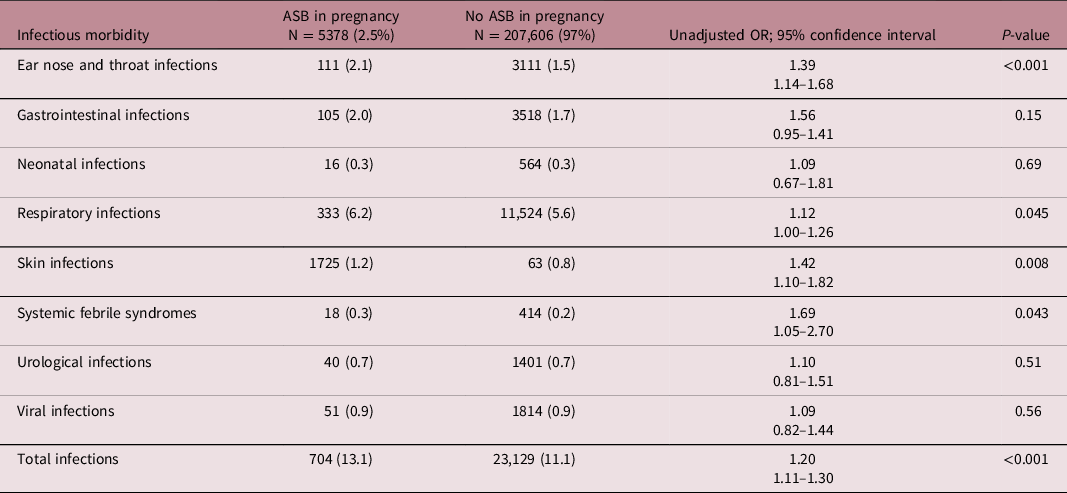
Table 4. Multivariable analysis for the association between ASB in pregnancy and offspring long-term infectious morbidity requiring hospitalizations

Discussion
Offspring to mothers who were diagnosed with ASB during their pregnancy had an increased long-term risk for neonatal and pediatric infectious morbidity, especially otorhinolaryngological, respiratory, and skin infections as well as nonspecific febrile disease. Additional infectious complications also had a higher prevalence in the maternal ASB category; however these findings were not statistically significant, possibly due to a limited number of cases.
The studied association has been previously investigated by Patrick. However, in her study there was no specification on whether the mothers were symptomatic or asymptomatic. Reference Patrick17 The findings in that paper concur with ours regarding infections in the newborn but lack data on long-term infectious morbidity. In addition, one recent study of pregnant women with symptomatic UTI showed increased otorhinolaryngological and respiratory infections in offspring. Reference Cohen, Gutvirtz, Wainstock and Sheiner18 It is therefore plausible to suggest that bacteriuria by itself, with or without symptoms, is associated with respiratory infections in early life.
Several mechanisms may explain the association between maternal bacteriuria and offspring infections, including mother–offspring transmission of pathogens, attenuated immune response to such bacteria, or late consequences of antibiotic treatment of ASB.
Maternal transfer of uropathogens was suggested by Patrick to be a major cause of infections after birth. In her study, maternal uropathogens were shown to be transferred to the fetus via amniotic fluid, umbilical cord blood, and placenta. Bacterial translocation was evident also in ASB. Patrick alluded that maternal uropathogens colonized fetal tissue and later on acted as pathogens. Reference Patrick17 Moreover, Patrick had observed an increased rate of ASB as well as clinical pyelonephritis in infants born to bacteriuric mothers, affirming her assumption.
In addition, Cooke et al. observed nonmaternal antibodies to E. coli in infants of mothers with bacteriuria, indicating that the infants were exposed to the pathogens. Reference Cooke, Hallock, Wurzel, Oski and Wallach19 Similarly, Brody et al. reported lymphocyte activation of the fetus in response to maternal urinary bacteria even in cases where mothers were asymptomatic. Reference Brody, Oski and Wallach20 All these reports suggest that higher infection rates in children to mothers with ASB may result from prenatal colonization.
Another assumption refers to a dysregulated immune response to uropathogens due to exposure in very early life, contributing later on to increased pathogenicity of such bacteria. Studies have suggested a number of mechanisms leading to the aberrant immune response which include immune tolerance and activation of specific cytokines. Reference Zhu, Zhang, Qu and Mu21–Reference Keski-Nisula, Katila, Remes, Heinonen and Pekkanen23 The fetal innate immune system is shifted to a more antiinflammatory reaction dominated by TH2 and TH17 cells as opposed to the proinflammatory TH1 response which is critical for combating infections in mature life. Hence, exposure to uropathogens in utero induces tolerance which in turn enables invasiveness of such pathogens in later life. Reference Padeh, Wainstock, Sheiner, Landau and Walfisch22 Moreover, chorioamnionitis increased the expression of interleukin-6, tumor necrosis factor-a, interferon-b, as well as other cytokines in uterus. These cytokines are aspirated by the fetus and cause lung injury potentially increasing susceptibility to respiratory infection. Reference Zhu, Zhang, Qu and Mu21
The influence of antibiotic consumption to treat ASB on infection in later life has only recently been researched. Currently, the most common treatment for ASB during pregnancy includes beta-lactams, nitrofurantoin, and fosfomycin. Reference Enbom24,Reference Pedler and Bint25 These antibiotics have specifically been shown to induce a sustained decrease of gut microbiota diversity. Reference Xu, Surathu and Raplee26 Antibiotics may change the microbiome in offspring. It is well established that gut microbiome is involved in inflammation and immunity. As an example, the pathogenesis of systemic inflammatory disease such as inflammatory bowel disease, multiple sclerosis, systemic inflammatory arthritis, asthma, and nonalcoholic fatty liver disease has been reported to be related to the gut microbiota. Reference Uchiyama, Naito and Takagi27 Furthermore, microbiota process gut content and thus create antigens that shape innate immunity. Reference Gensollen, Iyer, Kasper and Blumberg28 Therefore, changes in microbiome through exposure to antibiotics may have serious implications on the offspring’s health which are still not clear at this time.
In contradiction to the concept of the sterile womb, newer data support the possibility that fetal microbiota may develop in utero via the placental barrier or through ingestion of amniotic fluids. Reference Walker, Clemente, Peter and Loos29 Moreover, certain bacteria from the maternal gut may translocate to extra-intestinal sites and trigger immune reactions in the fetus. Studies have shown that memory CD4+ and CD8+ T cells can be identified toward the end of the first trimester in human fetal gut which in turn produce various cytokines in response to microbiota, thereby impacting immunity in the offspring. Treating ASB with antibiotics, which is according to standard practice, Reference Nicolle, Gupta and Bradley2,6,Reference Keating15 could therefore lead to altered microbiota in the mother and fetus with an abnormal immune response to pathogens, causing susceptibility to infections in the latter. Reference Nyangahu and Jaspan30 Indeed, it has also been found that the metabolites derived from microbiota can have a crucial influence on the airway cellular level that facilitate bacterial invasion which can lead to respiratory infection. Reference Arrieta, Stiemsma and Dimitriu31 These exogenous toxins and inflammatory mediators which are derived from maternal microbiota come in contact with fetal oropharynx and skin through the amniotic fluid and induce susceptibility to infection and inflammation.
Such a sequence of easier bacterial airway penetration and decreased immunity may explain the higher incidence of respiratory infections we saw in our study. In addition we found that, similarly to previous studies, Reference Sheiner, Mazor-Drey and Levy4,Reference Gehani, Kapur and Madhuri32 ASB was associated with significantly higher rates of CD. Such association may be explained by increased rates of PROM, preterm labor, and Intrauterine growth restriction associated with ASB. Reference Byna, Muvva, Kolli and Shaik33 It is established that off-springs born via CD have a less diverse microbiome than those born through vaginal delivery, possibly increasing susceptibility to infectious outcomes. Reference Reyman, van Houten and van Baarle34 Such an association between increased pediatric infections and CD has been reported previously. Reference Wainstock, Walfisch and Shoham-Vardi35
In summary, the findings in our study can be explained by altered microbiota, easier bacterial invasion, and attenuated immune and cytokine response leading to increase in clinical infection.
The key downside of this research is its retrospective design. As a result, we can suggest association but not causality. Another limitation is that the infectious cases we collected were only for hospitalized children, representing severe infectious cases. While the hazard ratio was significant but low (1.08), it relates to the spectrum of severe infections which require hospitalizations. Future studies should explore the association between ASB and childhood infections, which are mostly less severe, and managed in the community.
An additional limitation is the lack of information on whether or not any antibiotic was administered, its kind, dose and duration of the treatment, although it is a common practice at the Health Maintenance Organization from which the data were extracted. Thus, it is reasonable to assume that most cases with ASB were treated.
Indeed, environmental factors, possibly confounding the studied association, were unavailable and unaccounted for.
Another concern is that the prevalence of ASB in our sample was at the low range reported in the literature. Reference Whalley36 Since the reasons for the wide range are obscure, we cannot rule out a sampling bias. Reference Garnizov37
Our study’s biggest attribute is its large nonselective population-based cohort, which gives us confidence that our findings can be inferred to the general population.
In conclusion, our findings suggest that maternal ASB in pregnancy may have a major impact on offspring predisposition to infections requiring hospitalizations. Future research should focus on the mechanisms leading to such infections and to prospectively estimate the net effect of treating ASB, considering the offspring risk for infectious morbidity.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S2040174421000593
Conflicts of Interest
The authors report no conflict of interest
Statement of Authorship
Bluma Nae wrote the first draft of the manuscript. No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.







