According to FAO/WHO, probiotics are defined as living organisms that benefit consumer health when ingested in appropriate concentrations (Sanders, Reference Sanders2008). Most of the probiotics used by the food industry are lactic acid bacteria (LAB), because of their essential role in nutrition and food processing (Kechagia et al. Reference Kechagia, Basoulis, Konstantopoulou, Dimitriadi, Gyftopoulou, Skarmoutsou and Fakiri2013). LAB strains are usually incorporated in fermented food products as starter cultures and their beneficial/functional importance is associated mainly with their metabolism, such as substrate utilization and metabolite production capacities, as well as their direct beneficial properties (Oh & Jung, Reference Oh and Jung2015). The acceptance of probiotic strains and products by consumers increased when these bacteria were marketed as natural cultures that help in digestion processes and health (Kechagia et al. Reference Kechagia, Basoulis, Konstantopoulou, Dimitriadi, Gyftopoulou, Skarmoutsou and Fakiri2013). The use of food products containing probiotic bacteria covers a large part of the population, however therapeutic applications of LAB have a more limited scope (Foligné et al. Reference Foligné, Daniel and Pot2013).
In Brazil there is a growing market for probiotic food products. Brazilian consumers are becoming more aware about the health benefits of probiotic foods, and the food industry is following this trend, offering different alternatives (Pimentel et al. Reference Pimentel, Garcia, Prudencio, Nero, Cruz and Bersot2017). Dairy is the main sector associated with probiotic foods in Brazil (Granato et al. Reference Granato, Branco, Cruz, Faria and Shah2010) and the regulations for the production and certification are shared by the Ministry of Agriculture and the Ministry of Health; the first is responsible for registering and provide guidelines for production of the dairy products, while the second is responsible for assessing the beneficial properties and to regulate the usage of specific strains as probiotics.
In this review, we present the main aspects related to the characterization of probiotic and beneficial LAB strains with potential application in the dairy industry and the Brazilian scenario regarding this topic.
Definitions, benefits and selection criteria
Over the past 20 years research in the area of probiotics has progressed considerably, with significant advances in the selection and characterization of probiotic cultures, focusing on benefits to consumer health (Kerry et al. Reference Kerry, Patra, Gouda, Park, Shin and Das2018). Several studies characterise the beneficial activity of these microorganisms as well as their applications in the production of traditional and commercial fermented foods. Probiotic microorganisms are represented by different species, including bacteria, yeasts and moulds: LAB are the main explored organisms in the food industry, and strains from genera Lactobacillus, Bifidobacterium and Streptococcus are the focus of scientific studies and use in dairy processing (Reid, Reference Reid2015; Hossain et al. Reference Hossain, Sadekuzzaman and Ha2017). Among other bacteria, strains of Pediococcus, Propionibacterium, Enterococcus and Lactococcus are also widely explored due to their beneficial aspects (Fontana et al. Reference Fontana, Bermudez-Brito, Plaza-Diaz, Munoz-Quezada and Gil2013; Kechagia et al. Reference Kechagia, Basoulis, Konstantopoulou, Dimitriadi, Gyftopoulou, Skarmoutsou and Fakiri2013; Amara & Shibl, Reference Amara and Shibl2015; Kerry et al. Reference Kerry, Patra, Gouda, Park, Shin and Das2018).
Beyond the beneficial properties of LAB linked to their probiotic activity, these organisms are commonly used in fermented food products as starter or non-starter cultures and their importance is mainly associated with their metabolism, such as the use of substrates and generation of different products (Konings et al. Reference Konings, Kok, Kuipers and Poolman2000; Oh & Jung, Reference Oh and Jung2015). LAB are characterised by their spoilage activity, their organoleptic characteristics, the determination of their products and their interference in the survival of foodborne pathogens (Konings et al. Reference Konings, Kok, Kuipers and Poolman2000). These bacteria can produce substances with antimicrobial activity against spoilage and pathogenic microorganisms (Zacharof & Lovitt, Reference Zacharof and Lovitt2012).
Probiotic LAB are mainly used in applications related to the gastrointestinal tract, however, their use can be extended to the skin, oral and vaginal health (Foligné et al. Reference Foligné, Daniel and Pot2013). In the last decade reports have shown that even dead cells, or cell parts, of these microorganisms have a positive effect on the human immune system (Kerry et al. Reference Kerry, Patra, Gouda, Park, Shin and Das2018). Little is known about how probiotics influence host intestinal microbiota, therefore, the mechanisms for their beneficial effects are difficult to determine due to their multifactorial nature, which includes: gut microbiota modification, competitive adherence to the mucosa and epithelium, gut epithelial barrier strengthening and immune system modulation. All of these convey advantages to the host (Fontana et al. Reference Fontana, Bermudez-Brito, Plaza-Diaz, Munoz-Quezada and Gil2013). This occurs by means of protein and short chain fatty acid production, lowering of gut pH and nutrient competition that stimulates mucosal barrier function and immunomodulation (Ahire et al. Reference Ahire, Mokashe, Patil and Chaudhari2013). Immunomodulation has been the most studied issue and is verified by the capacity of probiotics to induce phagocytosis and IgA secretion, modifying T-cell responses, enhancing Th1 responses, and attenuating Th2 responses (Kechagia et al. Reference Kechagia, Basoulis, Konstantopoulou, Dimitriadi, Gyftopoulou, Skarmoutsou and Fakiri2013). Although the mechanisms of action of these microorganisms are not yet completely understood, their health benefits and safety for human consumption has growing scientific evidence. Probiotic effects tend to be strain specific: each strain can bring different benefits to the host (Fontana et al. Reference Fontana, Bermudez-Brito, Plaza-Diaz, Munoz-Quezada and Gil2013; Amara & Shibl, Reference Amara and Shibl2015). Figure 1 shows the mechanisms of activity of a probiotic bacterium, and Fig. 2 shows the benefits of probiotic bacteria in the host organism.
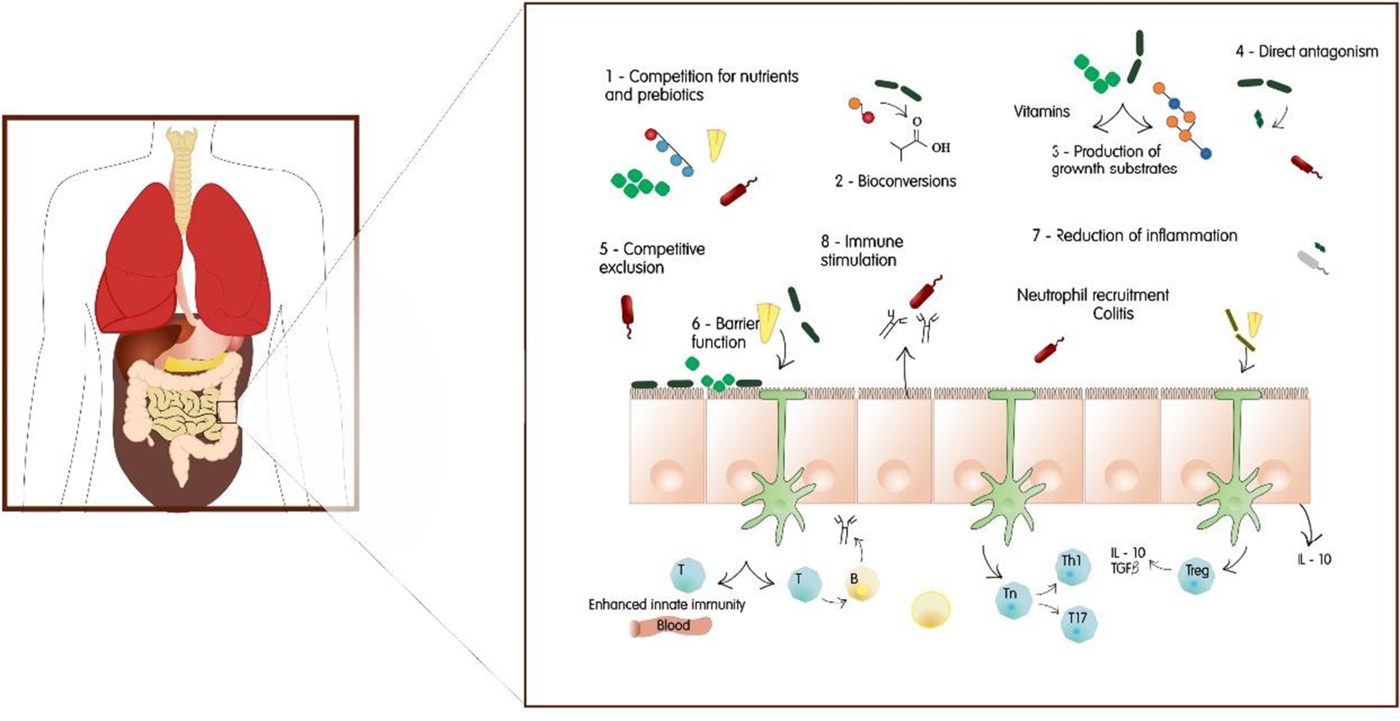
Fig. 1. Probiotic action mechanisms.
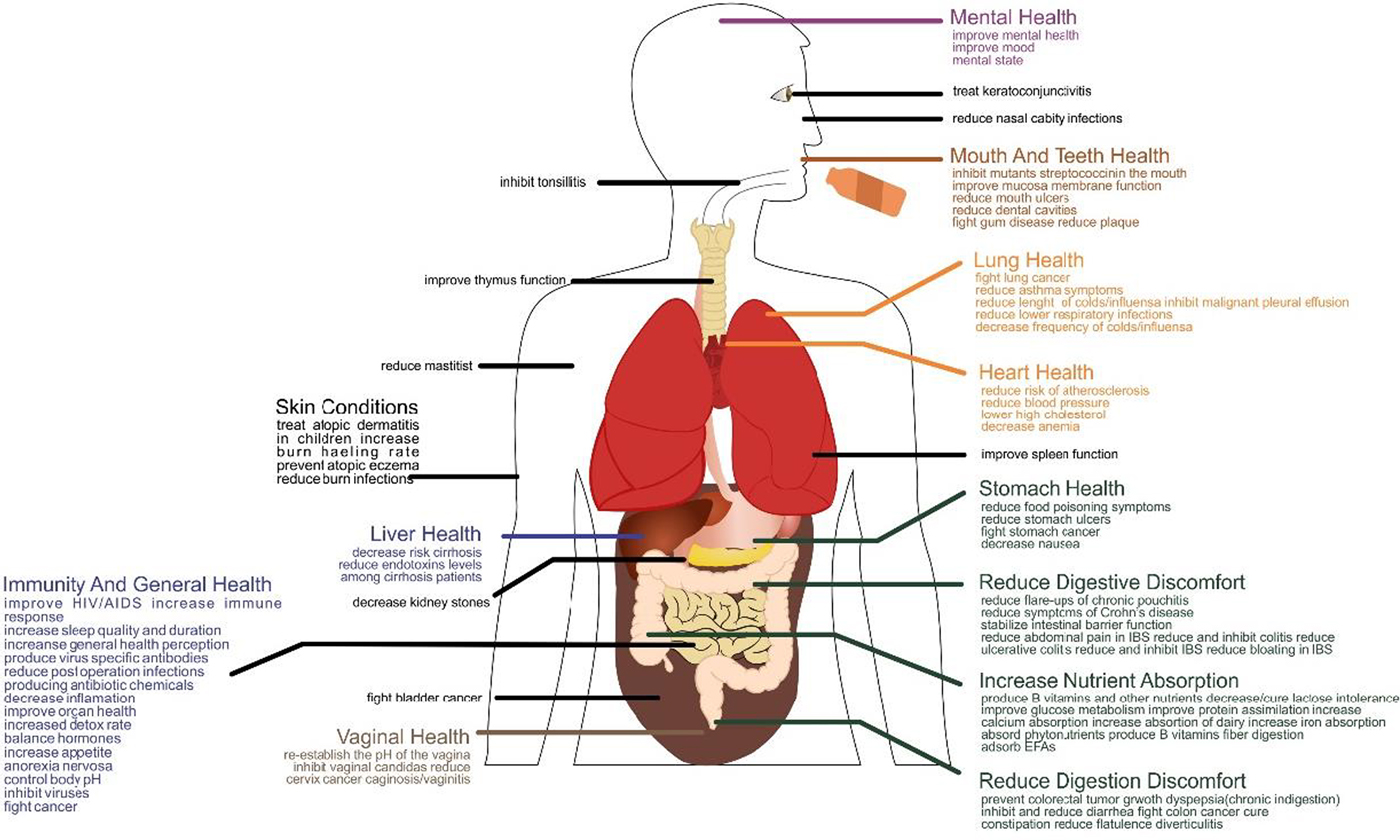
Fig. 2. Beneficial effects of probiotic bacteria.
The first step for obtaining novel probiotic strains is the enumeration and isolation of bacteria from a target group and the characterization of selected isolates by conventional and molecular approaches. Different culture media and protocols can be considered during this first step of screening, and conventional plating is mandatory, since it is necessary to obtain isolated colonies for further testing (Davis, Reference Davis2014). So, antibiotics, chemicals and incubation conditions are often considered to create selective conditions to improve the growth of target groups, mainly LAB (Colombo et al. Reference Colombo, Oliveira, Carvalho and Nero2014; Miranda et al. Reference Miranda, Carvalho and Nero2014). The isolates must be fully characterized by phenotypical and molecular methods, in order to identify their species and main characteristics. The criteria for selection of new probiotic species include features related to safety and efficacy, as well as technological aspects of the cultures to be used in the product composition. Finally, the approval also depends on depositing the culture in a registered collection of microorganisms, a systematic revision about the use of this species as probiotics, tests in animals and clinical controlled tests in humans (Hossain et al. Reference Hossain, Sadekuzzaman and Ha2017).
A potential probiotic organism must come from healthy animals, be commensal inhabitants of the intestine and be non-toxic and non-pathogenic (Salminen et al. Reference Salminen, Ouwehand, Benno and Lee1999). The efficacy or functionality of a strain is related to its ability to withstand the harsh conditions of the gastrointestinal tract (action of bile, gastric, pancreatic and enteric juices), as well as possession of antagonistic activity against resident pathogens (Salminen et al. Reference Salminen, Ouwehand, Benno and Lee1999; Kerry et al. Reference Kerry, Patra, Gouda, Park, Shin and Das2018). The selected strains may also present adequate technological properties, such as easy manipulation, fast growth in vitro, stability during storage (surviving in the final product at a predictable concentration and retaining function) and ability to multiply along with autochthonous microbiota of the host (Salminen et al. Reference Salminen, Ouwehand, Benno and Lee1999). Finally, animal models and in vivo tests are fundamental to the culture definition as probiotic (Hossain et al. Reference Hossain, Sadekuzzaman and Ha2017).
Despite the great interest of the industry in using probiotic cultures in food products to impart benefits to consumers, the use of these strains requires great caution due to the possibility of transferring their resistance or virulent genes to other microorganisms. The possibility of vertical transfer of genes between probiotic cultures and other bacteria is a concern in the food industry (Franz et al. Reference Franz, Huch, Abriouel, Holzapfel and Gálvez2011). Probiotic can have resistance to different antibiotics and carry genes related to these characteristics, increasing their potential virulence (Franz et al. Reference Franz, Huch, Abriouel, Holzapfel and Gálvez2011; Zhang et al. Reference Zhang, Jiang, Wan, Chen, Chen, Tao, Shah and Wei2016). Also, strains that possess virulence genes can express them in food, presenting a risk to consumers (Moraes et al. Reference Moraes, Perin, Todorov, Silva, Franco and Nero2012; Zhang et al. Reference Zhang, Jiang, Wan, Chen, Chen, Tao, Shah and Wei2016). This is particularly a concern for probiotic strains of Enterococcus spp. that harbour several virulence related genes and present a high potential of gene transfer.
Probiotics in the dairy industry
Probiotic cultures have been isolated from several food systems and animal environments, including dairy (Fontana et al. Reference Fontana, Bermudez-Brito, Plaza-Diaz, Munoz-Quezada and Gil2013). Dairy industries are often seeking for novel probiotic and beneficial strains, in order to develop novel dairy products to be offered to consumers as alternatives to keep their health and well-being (Reid, Reference Reid2015). Since the 1990s, fermented milk products have received significant attention because of the presence of these probiotic microorganisms in their composition; dairy products are the main vehicle for administration of probiotics at optimal concentrations for consumers (108–109 UFC per day, depending on the recommended diary ingestion for each product). Moreover, there is a long history of safe use of fermented dairy products, which would facilitate the security verification of these products (Fontana et al. Reference Fontana, Bermudez-Brito, Plaza-Diaz, Munoz-Quezada and Gil2013). Fermented milks are usually considered as ideal vehicles for probiotic strains, but other dairy products, such as fresh cheeses, are being increasingly used with this purpose.
Brazilian scenario for probiotics in the dairy industry: legal framework
The growing demand for dairy products in Brazil has led to an expansion of both the dairy industry and the producers responsible for providing quality raw material. In this context, the development of novel products, including probiotics, is a current trend: as the quality of raw milk produced in Brazil is improving, new fermented products are being developed. As consequence, Brazilian dairy industry offers to the consumers a panel of alternative probiotic dairy products, as described by Pimentel et al. (Reference Pimentel, Garcia, Prudencio, Nero, Cruz and Bersot2017) and Granato et al. (Reference Granato, Branco, Cruz, Faria and Shah2010), composed mainly of fermented milks (Table 1).
Table 1. Examples of some Brazilian dairy products that carry probiotic bacteria, available in retails sale for consumers

Source: Pimentel et al. (Reference Pimentel, Garcia, Prudencio, Nero, Cruz and Bersot2017) and Granato et al. (Reference Granato, Branco, Cruz, Faria and Shah2010).
However, this number is restricted by the historical difficulties related to Brazilian legislation.
Two Governmental institutions shared the regulations towards probiotics in Brazil. The Ministry of Health assess the beneficial and probiotic potential of the strains that will be used in the dairy production through the National Health Surveillance Agency (ANVISA), responsible for the registration of products and authorization of health-related companies, such as medicines, food and cosmetics. The Ministry of Agriculture (MAPA) is responsible for inspecting the dairy industries and registering the dairy products that will be produced under these inspection rules. Considering the different competences of MAPA and ANVISA regarding dairy products with claims of functionality, there is an agreement between these agencies. Thus, when a company has an interest in using functional and/or health claims in products that are of MAPA's competence, the company must first apply an evaluation form to request the allegation to ANVISA, which proclaims the decision to the company with a copy to the competent MAPA area. Likewise, when a new ingredient, including probiotic, is used in products of MAPA's competence, the company must request the evaluation of safety of use to ANVISA before the product is approved. After registration, MAPA is responsible for monitoring if the product maintains the quantities of probiotics described and sufficient for the claim.
ANVISA has published at least 12 regulatory rules in the last 20 years related to registration of new products and claims of functionality, including probiotics. They subdivide the products containing probiotics into 5 categories: medicines (Brazil, 2003), food (Brazil, 1999a, b, c, d), infant and enteral foods (Brazil, 2011a, b, c), supplements (Brazil 1999b, d) and supplements with added vitamins and minerals (Brazil, 2002). More recently, it has published the RDC no. 241/2018, which provides the requirements for proving the safety and health benefits of probiotics for use in food. It was published after a public consultation with the aim of defining more specific criteria for the evaluation of the safety and benefits of probiotics (Brazil, 2018). Together, these resolutions deal with the product registration process, applications for claims of functionality and probiotic properties, labelling and marketing about these characteristics. Added to this web of resolutions is the lack of an official unified list containing pre-approved species or strains of probiotic microorganisms and the impossibility of registering patents of living organisms in Brazil. Although the Agency's website contains a list of ten species of probiotic microorganisms, the ANVISA reports forecast the publication of an official list with strains approved as probiotics still for 2018. The General Food Management of ANVISA (GGALI) started the construction of the list and only three strains were considered suitable to be included in the Normative Instruction, namely: Bacillus coagulans GBI-30, Bifidobacterium lactis HN019 and Lactobacillus reuteri DSM 17938.
The complexity of the Brazilian regulatory system results in a low number of applications for registration of food with probiotics in Brazil and, mainly, a high number of rejections for probiotic claims relating to these products. Numbers provided by the Agency indicate that between 1999 and 2017, from the 211 applications for the registration of food containing probiotics, only 66 were deferred, while 100 were denied. The others are still under analysis. Only 12 products are registered in the Agency's General Food Management within the category of bioactive and probiotic substances isolated with claims of functionality and/or health properties. The main reasons for rejecting an application, according to the Agency, are the use of inappropriate methods for identification of the microorganism, lack of conclusive experiments on the safe use of this strain for humans and inconsistences between the claimed effects and the efficacy studies contained in the dossier.
The new legislation about probiotics in foods in Brazil foresees that the acceptance of the claimed benefit will occur after a positive conclusion regarding three areas: identification, proof of safety and proof of health benefits of the strain (Brazil, 2018). The identification must be carried out at the lineage level by means of phenotypic and genotypic tests and the deposit of the lineage into a collection of cultures recognized internationally. The proof of safety depends on the group to which the product will be administered and if the species already has security status considered in other international health agencies. Technical papers and scientific articles are considered for this issue. In general, in addition to the history of safe use, the absence of virulence and pathogenicity factors and non-production of metabolites that pose a risk to human health, as well as the safety of use in terms of antibiotic resistance, should also be considered. Finally, the health benefits should be communicated through evidence of functional or health property approved for the strain, which may be general or specific, although the criteria for each type of claim are not detailed in the RDC, but rather in complementary guides published by the Agency. General claims will require only a clinical study with evidence of the benefits of ingestion of the micro-organism. Specific claims will be for new claims, irrespective of micro-organisms, and will call for further studies to complete the claim. In addition to demonstrating the ability of the strain to survive the human digestive tract conditions, evidence of the beneficial effects on humans should involve a representative group of the population of interest, considering the claim, and considering the minimum amount suggested to obtain the benefit. Once the lineage has been approved for the claimed benefit, new products containing this lineage need not be subject to further approval.
Some publications from this Agency try to help companies to register new probiotics, as guides to fulfil requests and containing the steps needed for registration (available at http://portal.anvisa.gov.br/documents/3845226/0/Guia+Probioticos_Portal.pdf/e1bbf33e-719e-4f3e-84a0-7846bbe17972). However, the decision-making process is still subjective and has constantly changed since 2002, and more intensively since 2010. Some of these changes are positive, as they have made the evaluation of applications more specific and based on international guides and scientific knowledge. Regulatory predictability, with clear information to companies, and an agile decision-making process are essential requirements to ensure that the innovations demanded by consumers and generated by the industry come true.
Brazilian scenario for probiotics in the dairy industry: scientific studies on probiotic dairy products
Probiotic culture isolation and characterization is a prominent area of research in Brazil. Many Brazilian researchers have found in foods and dairy products, as well as environments and different regions, a potential source of new probiotic cultures that might be used in new products. Kefir grains are the focus of some of these studies. Lima et al. (Reference Lima, Souza, Albuquerque, Teixeira, Cavalcanti and Porto2017) assessed the probiotic properties of Saccharomyces cerevisiae isolated from kefir, demonstrating that some strains presented antagonistic activity against important pathogens, such as Klebsiella pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus subtilis, as well as important beneficial features. Vieira et al. (Reference Vieira, Cabral, Lima, Paschoalin, Leandro and Conte2017) tracked potential probiotic LAB strains isolated from Brazilian kefir grains with the ability to produce conjugated linoleic acid (CLA) during the fermentation of whole cow milk, a compound reputed to be of significant importance to health at low ingestion rates, leading to reductions in cancer incidence and severity of atherosclerosis. Although the bacterial production of CLA in foods is considered a technological challenge, the results were encouraging. Working with kefir, Zanirati et al. (Reference Zanirati, Abatemarco, Sandes, Nicoli, Nunes and Neumann2015) evaluated diverse cultures with probiotic potential, such as species of the genera Lactococcus, Leuconostoc, Lactobacillus and Oenococcus, and also demonstrated the possibility of using yeasts in combination with bacterial cultures. Leite et al. (Reference Leite, Miguel, Peixoto, Rosado, Silva and Paschoalin2013) reviewed several studies of isolation and characterization of microbiological, technological and therapeutic properties of kefir conferred on the symbiotic associations between bacteria and yeasts, and they highlighted the beneficial effects that could be provided by these co-cultures such as antimicrobial, antitumor, anticarcinogenic, hypo-allergenic and immunomodulating properties. In addition, they emphasized the importance of this dairy beverage in the daily food choices of diverse populations as well as the microbial diversity related to its origin.
Several studies show the importance of cheeses produced in Brazil as a fundamental source of probiotic microbiota. Martins et al. (Reference Martins, Freitas, Deuvaux, Eller, Nero and Carvalho2018) studied the bacterial diversity found in artisanal cheeses of the Amazon region (Pará) comparing between the dry and rainy seasons. Several genera were identified, predominant amongst them being Lactobacillus, Lactococcus, Enterococcus, Weissella, Pediococcus and Leuconostoc. In addition, this study showed the importance of current molecular approaches combined with culture-dependent methods in the detailed analysis of the microbial ecology of traditional cheeses from the Amazon region of northern Brazil. Campagnollo et al. (Reference Campagnollo, Margalho, Kamimura, Feliciano, Freire, Lopes, Alvarenga, Cadavez, Gonzales-Barron, Schaffner and Sant'Ana2018) selected native LAB from Brazilian artisanal cheeses produced with raw milk and characterized them as antagonist against pathogens. They also produced soft and semi-hard Minas cheeses, added the selected LABs and were able to identify strains of L. brevis and L. plantarum as potential beneficial cultures with anti-listerial activity. Studies like these show the importance of these beneficial cultures as an additional barrier to the growth of Listeria monocytogenes during storage of refrigerated soft cheeses, and at the same time the cultures shorten the semi-hard cheese ripening period when aged at room temperature. Acurcio et al. (Reference Acurcio, Sandes, Bastos, Sant'Anna, Pedroso, Reis, Nunes, Cassali, Souza and Nicoli2017) and Sant'Anna et al. (Reference Sant'Anna, Acurcio, Alvim, Castro, Oliveira, Silva, Nunes, Nicoli and Souza2017) isolated strains of L. plantarum and Pediococcus acidilactici from Brazilian artisanal cheeses and inoculated them in fermented milk. Feeding of the fermented milk to mice challenged with Salmonella typhimurium in controlled in vivo experiments demonstrated a protective effect, with less translocation and reduction of the pathogenic population and a consequent reduction of histological lesions, weight loss and mortality. Perin et al. (Reference Perin, Savo Sardaro, Nero, Neviani and Gatti2017) studied the bacterial ecology of artisanal Minas cheese and identified four genera: Lactobacillus, Lactococcus, Enterococcus and Weissella, with Lactobacillus being the most prevalent genus. In addition, the authors concluded that the microbiota of the Minas artisanal cheese is strongly influenced by its geographic origin as well as by the farm in isolation, which can be explained by the lack of standardization in the production procedures. In the study of Castro et al. (Reference Castro, Oliveira, Sant'Anna, Luiz, Sandes, Silva, Silva, Nunes, Penna and Souza2016), besides the artisanal Minas cheeses, lactic microbiota of water and raw milk were also identified and characterized. In this study, E. faecalis, L. lactis and L. plantarum strains were isolated from the cheese; L. brevis, E. pseudoavium, E. durans and Aerococcus viridans were isolated from endogenous starter cultures and described for the first time in the literature. The LAB identified in the sampled cheeses may inhibit the undesirable microbiota and contribute to the safety and flavour of the cheese. Paula et al. (Reference Paula, Jeronymo-Ceneviva, Silva, Todorov, Franco and Penna2015) isolated a Leuconostoc mesenteroides strain from buffalo mozzarella cheese and characterized its probiotic potential, identifying higher functional properties compared to other strains studied. Thus, the authors were able to state that the L. mesenteroides strain is a potential candidate for application in functional milk-based foods. Coalho is another very popular artisanal Brazilian cheese, from which Santos et al. (Reference Santos, Vieira, Buriti, Nascimento, Melo, Bruno, Borges, Rocha, Lopes, Franco and Todorov2015) isolated strains of L. rhamnosus and L. plantarum with beneficial potential. The same authors carried out a study characterizing the beneficial potential of E. faecium strains of Brazilian artisanal cheeses, characterizing them as bacteriocinogenic and thus excellent candidates to be used in probiotic products (Santos et al. Reference Santos, Vieira, Rocha, Nascimento, Lopes, Bruno, Carvalho, Franco and Todorov2014). Tulini et al. (Reference Tulini, Winkelstroter and De Martinis2013) also identified and characterized the bacteriocinogenic potential of a L. paraplantarum strain isolated from semi-hard Minas cheese. Overall, the results of this study indicated that L. paraplantarum is a potential probiotic and its production of bacteriocin may be an interesting feature for food applications. Finally, it is worth mentioning the importance of the production of goats and sheep in Brazil, as well as the production of dairy products derived from these species. Meira et al. (Reference Meira, Helfer, Velho, Lopes and Brandelli2012) isolated Lactobacillus strains from Brazilian regional sheep cheese, obtaining strains with interesting functional characteristics, mainly of the species L. brevis and L. plantarum as potential probiotic strains.
In addition to dairy products as main sources of probiotic cultures, milk itself (of various species) as well as the production environment can also be important. Moraes et al. (Reference Moraes, Abreu, Egito, Salles, Silva, Nero, Todorov and Santos2017) isolated and characterized LAB isolates from goat milk: L. mucosae strains attracted the attention of recent research due to their ability to adhere to the intestinal mucosa and inhibit pathogens in the gastrointestinal tract, both related to probiotic potential. Collectively, the results point to L. mucosae as a promising candidate for probiotic dairy products. Freitas et al. (Reference Freitas, Chuat, Madec, Nero, Thierry, Valence and Carvalho2015) studied the biodiversity of milk propionibacteria isolated from dairy farms in Minas Gerais, Brazil; bacteria belonging to this genus are known to be widely used in the maturation phase of Swiss-type cheeses, and in this study, the authors observed the considerable genetic diversity of wild propionibacteria in milk collected from different farms, with different origin (raw milk, silage, soil and pasture). In addition, different genetic profiles were found, indicating specific niches for each locality. These results show the dairy farms environment and milk production as excellent reservoirs for Propionibacterium strains and its future use as starter or probiotic cultures, as well as in the study of the prevention of cheese defects. Although not well reported, there are also studies showing the phenotypic and genotypic heterogeneity of probiotic species isolated from various foods (raw and pasteurized milk, meat products, cheeses and vegetables). Riboldi et al. (Reference Riboldi, de Mattos, Guedes Frazzon, d'Azevedo and Frazzon2008) and Gomes et al. (Reference Gomes, Esteves, Palazzo, Darini, Felis, Sechi, Franco and De Martinis2008) were able to investigate and determine the phenotypic and genetic diversity in enterococci isolated from different dietary sources. Genetic diversity was highest in E. faecium and E. faecalis isolates obtained from dairy products. Isolated samples of meat and vegetables offered the greatest genotypic variability.
Brazilian scenario for probiotics in the dairy industry: development of dairy products with probiotic cultures
The development of new food products becomes increasingly challenging as it seeks to meet consumer demand for products that are healthy and attractive. In Brazil, many studies propose the development of novel products based on Brazilian consumer taste, and exploring the beneficial strains isolated in Brazilian products, mainly from dairy origin. Fermented dairy beverages are the best known and easily produced: there are several studies demonstrating the proven benefits and quality of fermented milks added to probiotic cultures.
Weschenfelder et al. (Reference Weschenfelder, Paim, Gerhardt, Carvalho and Wiest2018) developed a kefir and evaluated its composition and antagonistic activity against S. aureus and E. coli. The authors investigated whether the kefir produced was able to meet the identity and quality standards of fermented milks, verifying the possibility of assigning a nutritional declaration. As a result, the authors were able to identify significant antagonistic activity against the tested microorganisms and suggest that further studies are carried out in order to explore their potential as a probiotic food and its inclusion in the diet of the population. Oliveira et al. (Reference Oliveira, Ribeiro, Oliveira and Vidigal2016) produced a frozen yogurt of goat milk with the flavour of cajá (Spondias mombim L.), a typical Brazilian fruit of the Cerrado and managed to identify the product as appealing to the food industry. Batista et al. (Reference Batista, Silva, Cappato, Almada, Garcia, Silva, Raices, Arellano, Sant'Ana, Conte, Freitas and Cruz2015) produced a probiotic yogurt with added glucose oxidase and evaluated its microbiological, physical–chemical and metabolic parameters. The product showed adequate viability of the dairy and probiotic cultures. With this study, the authors were able to conclude from a functional food perspective that the addition of glucose oxidase to probiotic yogurts may be an interesting technological option for small and medium dairy companies to enter the functional dairy market. Pimentel et al. (Reference Pimentel, Garcia and Prudencio2012) produced a probiotic yogurt with traditional LAB and a strain of L. paracasei ssp. paracasei as probiotic. In addition, inulin-type fructose of varying degrees of polymerization were added, and the results showed that such addition does not negatively affect the physical–chemical and microbiological characteristics of yogurts and their storage stability. In this way, it can be concluded that the selection of the type to be used depends on the manufacturer objective and the intended use. Mazochi et al. (Reference Mazochi, Matos, Val, Diniz, Resende, Nicoli and Silva2010) produced a probiotic yogurt with goat milk supplemented with Bifidobacterium spp., supplemented or not with strawberry flavour. The data enabled the product to be accepted by the Brazilian legislation. In the studies of Viegas et al. (Reference Viegas, Souza, Figueiredo, Resende, Penna and Cerqueira2010) and Oliveira et al. (Reference Oliveira, Sodini, Remeuf, Tissier and Corrieu2002), functional fermented milks were also produced from LAB cultures isolated from Coalho cheese and other lactic sources, respectively. Both studies confirm the recommendation of using probiotic cultures in association and are recommended for industrial elaboration of new probiotic fermented milks using Brazilian lactic cultures as starter or probiotics.
In addition to fermented milks, some studies demonstrate the potential of cheeses as potential carriers of probiotic cultures. Ferreira et al. (Reference Ferreira, Huang, Perrone, Schuck, Jan and Carvalho2017) aimed to develop a probiotic culture isolated from Amazonian cheese (Marajó cheese) made from raw buffalo milk for industrial applications using lyophilization and spray drying. Although the survival rate was similar among the samples evaluated after drying, the technological performance of the skimmed milk showed some differences. This study will help to direct further investigations on how to preserve probiotic LAB to produce spray dried primers that have a high number of viable cells and can then be used for industrial applications economically. Oliveira et al. (Reference Oliveira, Garcia, Egypto-Queiroga and Souza2012, Reference Oliveira, Garcia, Oliveira, Games, Pintado, Madureira, Conceição, Egypto-Queiroga and Souza2014) and Garcia et al. (Reference Garcia, de Oliveira, Queiroga, Machado and de Souza2012) added probiotic bacteria to Brazilian semi-hard goat cheese (Coalho) and concluded that goat cheese may be an interesting vehicle for probiotic L. acidophilus, L. casei subsp. paracasei and B. lactis. In addition, L. casei subsp. paracasei can be used as protective culture to slow the growth of S. aureus and L. monocytogenes in goat cheese. In similar fashion, Ribeiro et al. (Reference Ribeiro, Simoes and Jurkiewicz2009) have developed a Brazilian Minas cheese with L. acidophilus produced with retentate obtained by ultrafiltration of milk.
In addition to the conventional dairy products, such as cheeses and fermented milk, some studies propose the development of novel and innovative products for the area. Addition of honey produced by stingless bees to goat yogurt containing a L. acidophilus probiotic strain resulted in a successful incorporation, with satisfactory nutritional quality (Machado et al. Reference Machado, Oliveira, Campos, Assis, Souza, Madruga, Pacheco, Pintado and Queiroga2017). Figueiredo et al. (Reference Figueiredo, Passos, Passos, Lourenco, Araujo, Silva and Guimarães2015) have developed a powdered milk supplemented with L. acidophilus and, although during drying of milk most cells die, the authors were able to conclude that production of this novel probiotic food is feasible. Silva et al. (Reference Silva, Honjoya, Inay, Costa, Souza, Santana, Suguimoto and Aragon-Alegro2012) produced a chocolate pudding with added L. casei, characterizing this dessert as an excellent carrier of probiotic microorganisms. Antunes et al. (Reference Antunes, Grael, Moreno, Rodrigues, Dourado, Saccaro and Lerayer2007a, Reference Antunes, Marasca, Moreno, Dourado, Rodrigues and Lerayerb) have developed butter and buttermilk probiotics, respectively: both new products presented the desired requirements regarding safety and the number of probiotic bacteria.
Brazilian scenario for probiotics in the dairy industry: Acceptance of probiotic dairy products by consumers
It is understood that a product suitable to meet the needs and desires of the consumer has a greater chance of winning a certain audience and often the product is given value by the consumer who feels that the expectations are reached. As a result, more and more studies and research are needed with consumers, so that they are able to classify various foods as to their acceptability, and this certainly also applies to probiotic foods. Different Brazilian studies have demonstrated the acceptance of the Brazilian consumers for such probiotic dairy products.
Esmerino et al. (Reference Esmerino, Ferraz, Tavares, Pinto, Freitas, Cruz and Bolini2017) identified the main aspects involved in the perception of consumers regarding yogurts, fermented milk beverages and fermented. Although minor differences were observed, similar conclusions could generally be obtained from the study. The importance of such discoveries lies in helping dairy companies provide subsidies and guidelines for product redesign, marketing strategies, and improved communication between producers and consumers of different fermented dairy products. In the study of Machado et al. (Reference Machado, Oliveira, Campos, Assis, Souza, Madruga, Pacheco, Pintado and Queiroga2017), the authors achieved a successful incorporation involving honey and probiotic culture (L. acidophilus) into a new goat dairy product with satisfactory nutritional and sensory quality and added value to the market. Oliveira et al. (Reference Oliveira, Ribeiro, Oliveira and Vidigal2016) demonstrated the good acceptance and improvement of the sensory characteristics of a frozen goat milk yogurt flavoured with cajá. Costa et al. (Reference Costa, Balthazar, Franco, Marsico, Cruz and Conte2014) studied the taste perception of probiotic and conventional yogurts made from goat milk. Although this product is an excellent source of fatty acids, proteins and minerals, it is not well accepted by many consumers due to its typical flavour derived from the caprylic, capric and caproic acids present in this milk and dairy products: in this study, the authors were able to conclude that with regard to rapid repeated exposure, only six days were enough to significantly increase consumer familiarity with goat milk yogurt and probiotic goat milk yogurt. Thus, the authors could infer that increasing exposure sessions could be a strategy to increase acceptance of goat milk. Yogurt containing glucose oxidase from the study of Cruz et al. (Reference Cruz, Cadena, Castro, Esmerino, Rodrigues, Gaze, Faria, Freitas, Deliza and Bolini2013) through sensorial methodologies using consumer responses presented potential options for the characterization of food matrices with diverse sensorial sensations.
Barros et al. (Reference Barros, Rosenthal, Walter and Deliza2016) studied the attitude and opinion of consumers regarding different types of fresh cheeses. Products with different characteristics were used to encourage discussion among participants, including cheese with ‘no added salt’, the claim ‘contains probiotic microorganisms’ and products processed with goat milk. As results, the authors assumed that consumers would buy cheese with reduced salt if it did not have its flavour compromised; also, although the claim to contain probiotic in the product is not known to consumers, many have stated that they would buy the product. Garcia et al. (Reference Garcia, de Oliveira, Queiroga, Machado and de Souza2012) developed a Brazilian semi-hard goat cheese with addition of probiotic LAB and obtained the best sensory scores in the acceptance test, purchase intention and preference sorting test. In the study of Ribeiro et al. (Reference Ribeiro, Simoes and Jurkiewicz2009), the authors were able to develop a Minas pasta cheese with L. acidophilus produced with retentate obtained by milk ultrafiltration and had as a result of sensorial analysis a positive result by the acceptance of consumers. Studies such as these are likely to reveal important implications for marketing development strategies for probiotic foods.
Concluding remarks
There is little doubt between scientists about the benefits to human health of incorporating probiotics into food products. Specific probiotic treatments are also important for the prevention and control of diseases. Moreover, the increasing consumer awareness about the benefits of these microorganisms has meant that probiotic products have become increasingly popular and represent an important functional food market. In Brazil, despite the limitations and complexity of the regulations about probiotics, artisanal products and the dairy production chain are considered as relevant sources of beneficial strains, and have stimulated scientific studies to characterize them and also to develop novel dairy products, with good acceptance by the consumers.
The authors would like to thank CAPES, CNPq, FAPEMIG.





