Introduction
The conduct of successful, high-quality clinical research relies on enrolling and retaining individuals, who are invested in, understand and have trust in the clinical research process. Most measurements currently used to assess the overall success of a clinical trial are based not on participant-centered satisfaction but on appropriate data collection and how well the researchers adhered to regulatory processes such as appropriate conduct of informed consent [Reference Kost1]. However, many additional factors may contribute to successful research participant recruitment and retention including quality of care, cultural competency [Reference Otado2], and expertise of the research staff [Reference Smailes3].
Hospitals use surveys to document quality care, patient satisfaction, and institutional success. In the USA, the Centers for Medicare and Medicaid Services began to link hospital reimbursement to standardized scores using the Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) survey [Reference Pflugeisen4–Reference Geiger6]. The HCAHPS survey was created to allow objective comparisons between hospitals, create incentives to improve care, and enhance accountability and increase transparency in the quality of health care provided with public money [7]. Similar surveys may also be useful to determine participant satisfaction with regard to clinical trial experience.
Several studies [Reference Kost1, Reference Smailes3, Reference Verheggen8–Reference Kost10] have focused on satisfaction as it relates to participation in research studies and retention of participants throughout the duration of the clinical study. Verheggen et al. [Reference Verheggen8] conducted a survey in the Netherlands using personal interviews and telephone questionnaires and found that patient satisfaction was quite high. However, they found no relationship between perceived improvements in health and illness conditions and patient satisfaction with trial participation. Smailes et al. [Reference Smailes3] suggested that participant satisfaction is critical to the success of clinical research as patients and healthy volunteers are not required to participate in the clinical trials. Moreover, participants do not need to continue their participation once they have enrolled in a study but can withdraw without consequences for any reason. Kost et al. [Reference Kost1, Reference Kost9, Reference Kost10] hypothesize that improved understanding on the part of the research participant can enhance human subjects’ protection, enhance recruitment and retention, improve the quality of the research process, and increase public trust in the overall research enterprise. Thus, a participant-oriented approach to measuring satisfaction with the research process would be comparable to the current standard of evaluation of the clinical performance of hospitals and would be of use to clinical researchers.
Participation in research in general has been cited to be associated with satisfaction and their experiences while in a clinical trial [Reference Kost9, Reference Yessis11]. Studies indicate that the major barriers to participation in clinical trials, particularly with respect to African Americans and other racial and ethnic minorities, include a lack of awareness about trials, low socioeconomic status, mistrust, lack of communication, and lack of disease education [Reference Otado2, Reference Coakley12, Reference Harris13]. Further, these studies [Reference Coakley12, Reference Harris13] indicate that limited health literacy and minimal disease education contribute to the lack of enrollment of minorities into clinical trials. A recent study by Garza et al. [Reference Garza14] looked at willingness of minorities (African American and Hispanics) to participate in clinical studies. The study was a random telephone survey, and findings suggest that respondents’ top priorities for deciding to participate in the study were helping others, helping themselves, and gaining more knowledge about their disease. Reported barriers to participation have included length of the trial and the overall study design (e.g., randomization; chance of getting placebo instead of investigational drug) [Reference Martin15, Reference Jenkins16]. There is a growing interest among research investigators and clinical research staff to improve participants’ study experiences [Reference Kost1, Reference Smailes3, Reference Kost9, Reference Kost10, 17] and to increase participation by minorities. The Center for Information and Study on Clinical Research Participation (CISCRP) argues that it is important to determine participant satisfaction as few studies have examined the role participant satisfaction may play with regard to cultural diversity [17]. In addition, many of the survey studies [Reference Kost9, Reference Kost10, 17] that examined participant experience included only a small number of African American or other ethnic minority populations. It is, therefore, critical for researchers to find innovative ways to improve the overall study participant's experience, but more so with regard to African Americans and other racial and ethnic minority participants. Creating a trusting environment and ensuring understanding of the research process, particularly with regard to minority participants, must be a priority for clinical researchers.
Study Significance
The current study, therefore, seeks to determine the levels of satisfaction, experiences, and perceptions of research participants and to aid researchers to better engage research volunteers from all racial and ethnic populations in clinical trials, and thus reduce barriers, decrease drop-out rates, improve retention, and increase participation in clinical trials.
Methods
Study Participants/Population Demographics
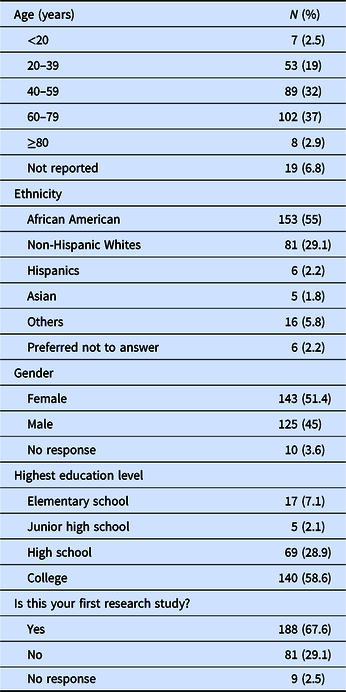
A non-probability volunteer sample of participants currently enrolled in research studies at the Georgetown-Howard Universities Center for Clinical and Translational Sciences (GHUCCTS) institutions (Georgetown University, Howard University and MedStar Health Research Institute) was obtained. The sites represented three clinical research centers within the Clinical and Translational Science Awards (CTSA) that includes two academic medical centers and a large health care system. Two hundred and seventy-eight participants or their surrogates (N = 278), 55% African American, and 29% non-Hispanic Whites, with average ± SD age of 52 ± 18 years, were consented and completed the participants satisfaction survey (see Table 1: Demographic Characteristics).
Table 1. Demographic characteristics of the study participants (N = 278)
Research Satisfaction Survey Development
A self-administered, institutional review board exempt study, survey on satisfaction and perceptions of research participants in clinical and translational studies was developed based on existing and validated survey instruments (CISCRP [17]). The CISCRP instrument is a well-known validated questionnaire that has been used to conduct several national and international surveys on public perception and participation experiences in clinical research over the past years and in various populations [Reference Williams18, Reference Felemban19]. The additional questions in our survey were minimal and adapted from another validated instrument [Reference Smailes3]. The study questions were pilot tested with 15 research participants (i.e., five participants from each of the three GHUCCTS clinical research sites) to evaluate their feasibility. The survey was then revised to reflect the feedback based on the pilot test. The final survey included a 15-item Likert-type questionnaire. It focused on three domains: (a) satisfaction with staff delivery of care, (b) satisfaction with environment, and (c) satisfaction with center operations. Another set of questions reflected “overall experiences and perceptions.”
Study Procedures
The survey was administered to study participants who volunteered to complete it during a visit within the research study. Further, the participants acknowledged whether this was their first research study or if they had participated in a research study previously. Study participants could complete the questionnaire either online directly into the REDCap database or on a paper questionnaire. The data from the paper questionnaires were then entered into the Redcap database by staff. The survey responses were completely anonymous.
Data collection took place from July 2016 to February 2017. During this period, 1692 participants were enrolled in research studies among the three GHUCCTS institutions. All 278 (16.4%) who were approached for this study agreed to complete the survey. Study data were collected and managed using REDCap electronic data capture tools hosted at Georgetown University [Reference Harris20]. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and, 4) procedures for importing data from external sources.
Data Analyses
Semiquantitative and qualitative data analysis methods were used for the analysis of survey data. Quantitative and semiquantitative measures were analyzed using appropriate summary statistics such as mean, median, counts, chi-square test, and percentages. Thematic content analysis was used to analyze qualitative survey responses. Analyses were done using SPSS version 22 (IBM Corp., Armonk, NY, USA).
Results
The demographic characteristics of the study population are shown in Table 1. One hundred forty-three participants (143) or 51% of the subjects studied were female. Fifty-eight percent of the participants were college-educated. Participant ages ranged from 16 to 93 with an average age of 52 ± 18 years. One hundred fifty-three (55%) of the participants were African Americans, and 81 (29%) non-Hispanic Whites. One hundred eighty-eight (68%) were first-time research study participants.
During the period when this survey was conducted, the study participants and research staff including the research nurses and study coordinators at Howard University were predominantly African American. At the Medstar and GU centers, the participants were 56% African American, 32% White, and the remaining were of other ethnicities, while the research staff at these centers were about 30% African American, 52% White, and the rest other ethnicities.
Table 2 presents results of the three key domain areas on research participant satisfaction with “Staff delivery of care,” “Environment,” and “Center Operations by ethnicity.”
Table 2. Results of responses on satisfaction on three domains by ethnicity. Data are number (%)
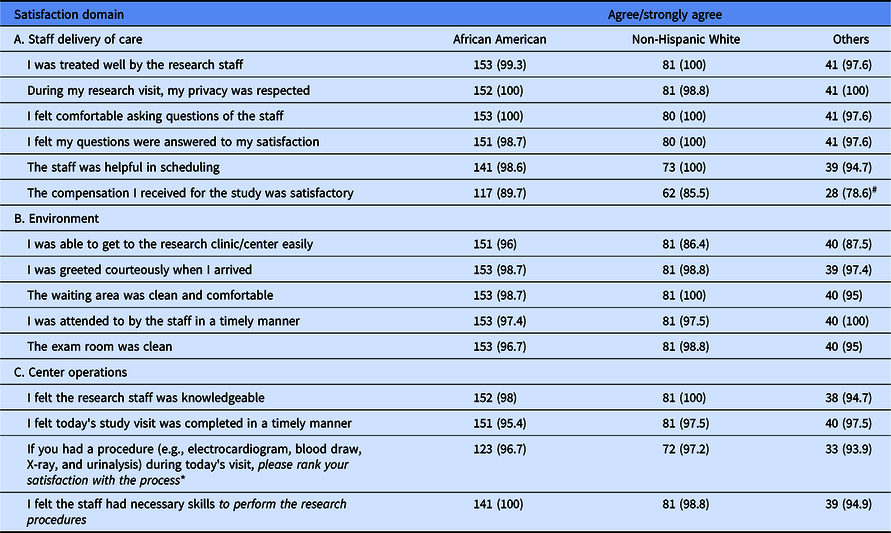
* Responses to this question were rated as very satisfied/satisfied.
# Statistical significance difference in response among the ethnic groups at the 5% level assessed by the chi-square test.
The results show that overwhelmingly a majority of the respondents were satisfied with all aspects studied in relation to their clinical trial participation regardless of ethnicity. The data indicate that over 90% either agreed or strongly agreed with each statement except for the compensation received. Although data are not shown, age, gender, or educational level did not impact the level of satisfaction.
Respondents Knowledge and Experiences on the Specific Study of Their Participation
In this section, the questions to the respondents were focused on the specific study they were currently participating in. One hundred eighty-eight participants (67.6%) stated that this was their first research study, while 81 (29%) respondents stated they had previously participated in a research study. Forty-two of the participants stated that this visit was their initial visit to the center for the study in which they were currently enrolled, while 230 participants responded that they were at the center for a follow-up study visit.
Fig. 1 indicates that there is a difference in responses between first-time research participants and those participants who had previously participated in a research study with regard to “Knowledge of length of study.” For the combined data, the results showed a significant difference in “Knowledge of length of study” between “first-time research participants” and “repeat-participants” (73.9 first-time versus 53.8% repeat, p = 0.002). While stratifying by ethnicity, there was a difference only in the African American cohort, (64% first-time versus 46% repeat, p = 0.002), but no difference was observed in the non-Hispanic White cohort.

Fig. 1. Knowledge of length of study by first timers vs previous participants by ethnicity.
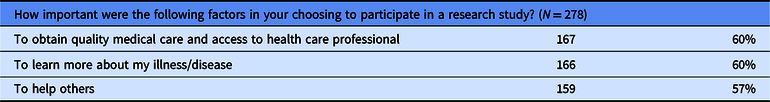
The data in Table 3 reflect the important factors participants use to make a decision when choosing to participate in a clinical trial. The participants in this study were asked to choose which factors were important to them.
Table 3. Important factors in choosing to participate in a clinical trial
Respondents Study Perceptions
Survey items related to participants’ overall experiences included a combination of both qualitative and quantitative questions. Most of the quantitative questions were followed by open-ended questions, which a majority of respondents left blank or were answered by only a few participants. Findings indicate that the majority (87%, N = 278) of the participants’ expectations were met while participating in the study (see Table 4).
Table 4. Overall experience and perceptions

The percentages may not add up to 100% due to missing data (i.e., non-responses).
Most (71.6%) of the participants stated that their overall experience was excellent with the research study in which they were enrolled; 23.7% stated their experience was very good. Further, 82% indicated they would be willing to participate in a future clinical study, while 13.7% stated that it depends. Those who responded to the “It Depends” open-ended question stated that their future participation would depend on the nature and objectives of the study. Additionally, the study findings show that 91% would recommend a family member or friend to participate in a clinical research study.
When asked, on the one hand, what they liked most about participating in a research study, 21.5% (n = 60) cited “friendliness and expertise of the staff,” 21.1% (n = 59) stated “knowledge of their disease,” and 11.5% (n = 32) of respondents cited “contribution to science.” On the other hand, when asked what they liked least about the study, the respondents cited: being uncomfortable with procedures such as blood draws and barriers to transportation and parking. Regarding compensation, 62% (n = 117) of the first-time participants agreed that the “compensation received was satisfactory,” compared to 74% (n = 60) of participants who had previously participated in research studies (i.e., repeat-participants).
Discussion
Patient surveys have been used in the hospital setting for many years, but there have been few surveys conducted to determine participant satisfaction in clinical research. The purpose of the current study was to determine if most research participants are satisfied with their participation in the studies and with the clinical research process in general, including research staff. In addition, the study examined what factors motivate participants in choosing to participate in a clinical research study. These findings will help to inform the research community regarding how the research participants rate their experiences, what factors motivate them to participate in clinical research, and what barriers they perceive to participating. Our study findings demonstrated that a majority of research participants rated their experience as highly favorable even among those who had never participated in a previous clinical research study. Further, in our sample, there was no difference in the favorable ratings as determined by race/ethnicity, age, gender, or education.
This study not only demonstrated the willingness of African Americans to participate in clinical research, but also whether they were satisfied with their experience, willing to participate in future studies, and would also recommend a friend or family member. A recent study by Garza et al. [Reference Garza14], using a population composed completely of minorities, reported that African Americans and Latinos were not only willing to participate in research but also 50% of the African Americans and 53% of the Latinos were willing to take medicine as part of a research study. These findings are contrary to perceptions on lack of participation and unwillingness of African Americans to participate in clinical trials [Reference Otado2, Reference Coakley12].
Further, our observations revealed that the main reasons or factors motivating individuals in choosing to participate in a clinical trial were to increase knowledge about their own disease, to contribute to science, and to help others, respectively. These findings are supported by the existing literature [Reference Geiger6] and are consistent with the findings reported by Garza [Reference Garza14], Kost [Reference Kost1, Reference Kost9, Reference Kost10], and Smailes [Reference Smailes3]. Our study findings showed that a majority of the participants, regardless of ethnicity, stated they would participate in future studies and would recommend participation in a clinical trial to a family member or friend. These findings are consistent with those reported by CISCRP [17].
Financial compensation has also been reported as a major motivation for participation in studies involving healthy volunteers [Reference Kost1, Reference Slomka21]. Slomka et al. [Reference Slomka21] indicated that financial compensation was a major motivation to participation in studies involving drug users in an HIV study. CISCRP study [17] also showed that financial compensation was one of the major motivations for participation. Our study findings, however, indicated that financial compensation was not a motivation for participation. The demographics of the study population and the disease being studied may play a part in determining the role financial compensation plays in the recruitment and retention of participants [Reference Kost9].
One interesting finding from our study was that first-time participants appeared to have a better knowledge of the length of study than individuals who had previously participated in a research study. Kost et al. [Reference Kost9] reported that approximately one-third of their study participants did not fully understand the consent document. Among the African American population, our data show that knowledge of the length of study was significantly less for those individuals who had previously participated in a research study. These findings suggest that individuals who are enrolling in a study for the first time may be more attentive, curious, and eager to know the study procedures and what is required or expected of them, whereas those who have previously participated are probably more relaxed as they feel more comfortable with the research process. The 2017 CISCRP survey [17] reported that understanding of the consent forms amongst minorities, particularly Hispanics, was less than for non-Hispanics. Similarly, Kost et al. [Reference Kost9] reported that approximately one-third of their study participants did not fully understand the consent document. These results imply that researchers and study teams should be more aware of appropriate recruitment and retention strategies when working with African American and other racial and ethnic minorities. Keeping constant contacts with participants throughout the duration of the study has been reported to work well for the African American population [Reference Otado2].
While reasons for withdrawing from a clinical trial may be due to many factors, dissatisfaction with a trial or with the study staff could be a major issue. Factors influencing participant satisfaction may be the degree of coordinator training and the expertise of the clinical research staff. The level of coordinator training and expertise may have a direct correlation with participant satisfaction and overall understanding of the research protocol. Perhaps research participant satisfaction is a direct reflection of the level of research coordinator skills and the degree of satisfaction with their own role as research coordinators [Reference Leiter, Harvie and Frizzell22, Reference Speicher23]. Thus, the degree of satisfaction with the research staff and understanding of the trial itself are crucial to reducing drop-out rates and increasing compliance with study procedures. Participants’ understanding of the research process, study procedures, and satisfaction with the research staff are key to increasing participation and retention in clinical trials, probably more so among African Americans and other racial and ethnic minorities due to past medical experiences and poor medical treatment [Reference Coakley12, Reference Corbie-Smith24].
One notable limitation of this study is that it did not address the congruence between ethnicity of the study recruiter/investigator and the participants. This congruence has been documented by some researchers [25] to be a factor in successful recruitment and enrolling participants into clinical trials, particularly with regards to the racial and ethnic minority populations.
Another limitation is the convenience sampling of the participants. This limitation is minimized because the participants reflect a true representation of the population served. Another limitation is the potential response bias from administration of the survey at the research sites. Future studies of this nature could use a third party to conduct the survey offsite to limit response bias.
In conclusion, the findings from this study suggest that positive attitudes of research staff and study coordinators can create motivation for potential research participants to view clinical research participation in a positive light and hence increase willingness to participate regardless of their race/ethnicity.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cts.2020.20.
Acknowledgments
We also acknowledge the following study personnel: Amy Loveland, Debra Ordor, and Sarah Vittone for their assistance with data collection of the paper surveys from the research participants.
This project has been funded in whole or in part with Federal funds (UL1TR000101 previously UL1RR031975) from the National Center for Advancing Translational Sciences, National Institutes of Health, through the CTSA Program, a trademark of Department of Health and Human Services, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise.”
Disclosures
The authors have no conflicts of interest to declare.







