Introduction
The interactive process of obtaining informed consent is a critical event for most human subjects’ research, but informed decision-making could be threatened if materials are difficult to read. Recent regulatory updates have prompted the addition of even more potentially complicated content to informed consent forms (ICFs), and our study aimed to determine the impact of those new requirements on the readability of ICFs at an academic institution.
Scientific breakthroughs often depend on data from human subjects which often requires the explicit consent of the participants in those studies. The consent process is to be conducted in a manner that does not perpetuate biases, barriers, or inequities that could skew the generalizability of the research or limit opportunities for everyone to benefit. Thus, regulatory guidelines and requirements exist to help ensure that decisions to participate in a research study are devoid of undue coercion or inducements and that all potential risks and benefits are clearly and equitably conveyed to all eligible individuals.
The 1979 Belmont report was written by the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research [1] to establish ethical guidelines to protect human research subjects. The US Department of Health and Human Services requires investigators to obtain “legally effective informed consent” of participants in human subjects’ research [2]. These regulations further state that participants must be given information in language that is understandable by the participant [2]. The ICF must also contain the basic information required in 21 CRF 50.25 (a). The recently revised Common Rule [Reference Menikoff, Kaneshiro and Pritchard3] also stipulates that all ICFs must include a key information section, which is a concise summary statement that explains the research to potential participants in a way that is clear and easy to understand.
The complexity of clinical research protocols and medical jargon included in ICFs can be a major barrier for vulnerable populations, including those with limited health literacy skills. US surveys report that over 80 million adults have limited health literacy skills, [Reference Berkman, Sheridan, Donahue, Halpern and Crotty4,Reference Kutner, Greenberg, Jin and Paulsen5] and only 12 out of 100 have proficient health literacy skills. Healthy People 2030, the nation’s 10-year health objectives, expanded its previous definition of health literacy to include both personal and organizational perspectives. This was a shift from previous definitions which focused only on an individual’s capacity to find and use health information. A new definition highlights organizational accountability by defining organizational health literacy as “the degree to which organizations equitably enable individuals to find, understand, and use information and services to inform health-related decisions and actions for themselves and others” [6]. This definition emphasizes the role that health care professionals, including researchers, have in addressing health literacy challenges.
An interdisciplinary investigative team at the University of Arkansas for Medical Sciences (UAMS) previously reported on the development of an ICF template [Reference Hadden, Prince, Moore, James, Holland and Trudeau7] using plain language writing techniques. We define plain language as that which is optimally readable, understandable, and actionable. The original template improved the readability of ICFs, with forms written using the template scoring three grade levels lower (better) than those written without it. In the study described here, the template was updated to comply with the revised Common Rule. Plain language writers added specific language to address collection and use of biospecimens and genetic information. In addition, a “Key Information” section was added. While there was not definitive guidance from any regulatory body as to specific content that must be included as key information, the team of writers and local IRB personnel agreed to include the voluntary nature of joining the study, total maximum length of time a participant would be enrolled, major reasons to consider joining, major reasons that may lead to a decision not to join, and a brief description of study activities. The most current template is available at https://healthliteracy.uams.edu/health-literacy-research/resources/. Of note, only the most current template is housed on this site and may differ from the templates described in this study. The current study aimed to evaluate the impact of ICF template updates on the readability to determine if additional requirements of the revised Common Rule are likely to serve as a barrier or facilitator to subjects’ likelihood of reading ICFs with ease.
Methods
The purpose of this study was to assess how the requirements of the revised Common Rule have changed the readability of institutional ICFs at one academic institution. We compared readability longitudinally, independent of template use, during three periods of time: before a template was introduced (P1), after the introduction of the original template (P2), and after the introduction of the most current template (P3). We also assessed changes in ICF readability based on template use during the development of ICFs, including the use of the original (T1) and updated (T2) plain language templates along with those written without a plain language template (NT). In addition, we assessed the rate of ICF template adoption during P2 and P3.
We collected ICFs from investigator-initiated studies submitted to the UAMS IRB. To assess readability, staff from the UAMS Center for Health Literacy used standardized processes. For each ICF, 600-word samples were selected from the beginning, middle, and end of the text. Selections were made by a single staff member to ensure consistency. Staff then cleaned each sample to optimize the accuracy of results. Examples of tasks in the cleaning protocol include removal of bullet points and extraneous punctuation (e.g., removing the period from “Dr. Smith” to avoid the software interpreting it as the end of a sentence). Cleaned samples were loaded into Seven Formulas software (Micro Power & Light Co., Dallas, TX, USA) to generate readability statistics from three validated formulas: Flesch−Kincaid [Reference Kincaid, Fishburne, Robert, Richard and Brad8], SMOG [Reference Mc Laughlin9], and Fry Graph [Reference Fry10]. Scores from the three formulas were then averaged to arrive at a mean readability score for each ICF. Each numerical mean score, presented as a grade level rounded to the nearest tenth (e.g., grade 9.1) was categorized into levels of difficulty (“easy,” “average,” or “difficult”) [11].
Mean readability scores for the ICFs approved during time periods P2 and P3 were statistically compared with those from P1 using one-way analysis of variance (ANOVA) via SPSS (IBM Statistics, v.21). The mean readability scores for all ICFs that were created using the original plain language template (T1) and the revised plain language template (T2) were also statistically compared with those that did not use a template (NT) using ANOVA via SPSS.
Results
Mean institutional readability scores improved across the three time periods studied (Table 1). During P1, average readability across ICFs was grade 11, placing the mean in the “difficult” category. While the institutional mean was improved during P2, with reading demand lowered by one grade level to grade 10, the institutional mean remained in the “difficult” category. During the final time period studied, P3, additional improvements resulted in a campus-wide mean readability score of grade 9, shifting the institutional mean into the “average” level of difficulty. Further, the proportion of ICFs scored as “difficult” decreased substantially over each time period (P1: 82.1%, P2: 46.3%, P3: 21.1%).
Table 1. Study ICFs based on time period

ICF, informed consent form; IRB, Institutional Review Board.
Time period before a template was introduced (P1), time period after the introduction of the original template (P2), and time period after the introduction of the most current template (P3).
In examining the rate of adoption of each iteration of the template, we found that during P2, just over half (52.1%) of ICFs used the available template. During P3, template adoption had substantially improved with 71.9% of approved ICFs developed using a plain language template.
While the readability of ICFs improved for the institution overall between each sampling period, we observed the greatest improvements in readability when a plain language template was used. When including all three time periods and focusing exclusively on whether a template was used, and if so, which version, we found that use of either template (T1 or T2) yielded significant improvements over NT.
While both samples of ICFs using T1 and T2 were assessed in the “average” category (grade 8), the sample developed without a template registered as “difficult” at grade 11. Thus, the use of either template (T1 or T2) yielded statistically significant improvements as compared to NT (see Table 2).
Table 2. Study informed consent forms (ICFs) based on template used
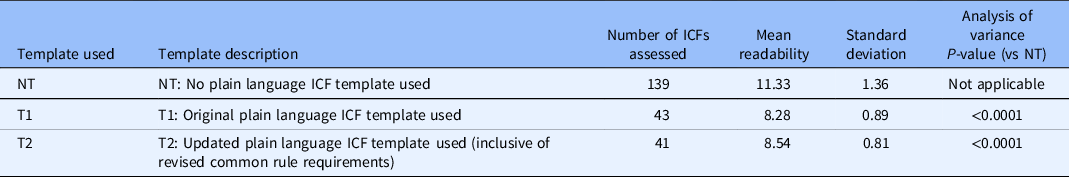
The readability of ICFs developed using T1 was grade 8.3 (average), and for those developed using T2, the readability was grade 8.5 (average). Thus, the template revisions made to accommodate new requirements of the revised Common Rule did not significantly impact the readability of ICFs (see Table 2).
Conclusion
Informed consent is fundamental to the ethical conduct of human subjects’ research. While consent forms are not intended to stand alone, but rather to serve as tools used during an informed consent process, they are indeed intended to support autonomous decision-making by prospective participants. Our results align with previous studies [Reference Emanuel and Boyle12,Reference Munley, Buser, Gaudreau, Breault and Bazzano13] which have demonstrated that many consent forms are written at difficult reading levels. The complexity of consent forms could impact understanding of a study’s purpose, structure, risk, and benefits by potential human subjects. This could limit study participation and undermine efforts to ensure informed decision-making.
Results of this and previous studies confirm that a plain language informed consent template can lower reading demands. In addition to the ethical principles described above, addressing readability is an important step toward expanding inclusivity in research. Populations with known health literacy challenges include certain minority groups and older adults [Reference Kutner, Greenberg, Jin and Paulsen5], and inclusion of these groups in research is a focus of many institutions as they align their work with national priorities to improve health among these groups [14,15].
Our study confirmed that the addition of new sections to an ICF template did not adversely impact readability of ICFs. Those new sections address biospecimen collection and storage and offer readers an introductory “Key Information” section to highlight important study details. While these are seemingly important details to help facilitate informed decision-making, we recognized that new content could impact readability. This review suggests that this new content did not negatively impact readability.
Readability results should be interpreted with caution. While the study team used standardized protocols to select samples from each ICF included in the assessment, it is possible that results could be different if alternative samples had been chosen. Further, readability at a desirable level does not ensure that prospective human subjects understand the content within an ICF, in its entirety or within specific sections.
As noted, the readability of ICFs has improved at our academic medical center over time. While it is clear that increased use of a plain language template contributes to these gains significantly, there may be additional factors that support these improvements including an ongoing effort to provide health literacy training to research personnel and the availability of plain language writers to assist investigators with the preparation of ICFs.
The study team will continue outreach to investigators to underscore the importance of providing ICFs and other study materials in language that prospective and enrolled participants can understand and use in decision-making. Future studies may explore the impact of readability on study enrollment and retention, particularly among groups underrepresented in research, and explore the role of a web-based consent builder in further equipping investigators to produce plain language consent documents.
Acknowledgements
Authors wish to acknowledge support from the UAMS Center for Health Literacy and the UAMS Institutional Review Board who contributed to this work with their respective subject matter expertise.
The project described was supported by the Translational Research Institute, grant UL1 TR003107 through the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosures
The authors have no conflicts of interest to declare.




