To the Editor—The Foundation for the Accreditation of Cellular Therapy and the American Association of Blood Banks publish guidelines to ensure the quality and safety of hematopoietic stem-cell (HSC) products. 1 , 2 These HSC products are generally cultured after procurement by the collection facility and following processing at the transplant center. Reported contamination rates of HSC grafts range from 1% to 45%.Reference Kamble, Pant and Selby 3 – Reference Patah, Parmar and McMannis 5 The clinical significance of infusion of contaminated HSC products is unclear. When fresh products are used, contamination is often not identified prior to HSC infusion. Bacterial contamination is not an absolute contraindication to HSC infusion, as options are limited following a myeloablative preparative regimen. In a review of 12 studies, 91% of contaminated grafts contained bacterial species of low pathogenicity (eg, Staphylococcus epidermidis and Propionibacterium acnes). Of 26 patients who received grafts contaminated with highly pathogenic bacteria (eg, S. aureus), none developed symptoms or had a positive culture matching an organism found in the HSC graft.Reference Kamble, Pant and Selby 3 In prior reports of infections putatively caused by graft contamination, confirmation that the graft was the source of infection was based solely on the finding of identical species.Reference Webb, Coral and Andersen 6 , Reference Lazarus, Magalhaes-Silverman, Fox, Creger and Jacobs 7 Contrary to these prior reports, we present a case of catheter-related bloodstream infection with methicillin-susceptible S. aureus due to a contaminated HSC graft in which pulsed-field gel electrophoresis (PFGE) confirmed that the graft and patient isolates were identical.
A 15-year-old male presented for hematopoietic cell transplant (HCT) for hypodiploid B-cell acute lymphoblastic leukemia (B-ALL). His history included osteosarcoma of the proximal right tibia, for which he had completed treatment with chemotherapy and limb-sparing resection two years prior to this admission. At the time B-ALL was diagnosed, local recurrence of osteosarcoma was also discovered in the distal right femur. He began therapy for both cancers per the Children’s Cancer Group protocol 1941 with modifications as appropriate. He underwent a right transfemoral amputation and achieved remission of osteosarcoma.
After achieving complete remission of B-ALL, he underwent a 10/10 HLA–allele matched unrelated donor HCT. Preparative regimen per Children’s Oncology Group protocol AALL1331 included fractionated total body irradiation, thiotepa, and cyclophosphamide. Graft-versus-host disease prophylaxis included tacrolimus and methotrexate. Antimicrobial prophylaxis included posaconazole, valacyclovir, pentamidine, and levofloxacin. Marrow was collected at an outside collection center approximately 24 hours prior to infusion, and the product remained at room temperature. On day 0, the patient received the fresh donor marrow via the red lumen of his tunneled double-lumen central venous catheter (DLCVC). Infusion was complicated by hypertension and bradycardia, managed with furosemide and hydralazine.
Approximately 24 hours after completion of HCT, the patient developed a fever of 38.3°C associated with tachycardia but no other signs of sepsis. Blood cultures from all lumens of the DLCVC and the single-lumen CVC were obtained, and antibiotics were broadened to vancomycin plus cefepime. On day +2 after fever onset, the team was notified that the culture of the HSC product performed just prior to infusion had grown a single colony each of Micrococcus and Staphylococcus aureus. Blood cultures from the red lumen of his DLCVC were positive for Staphylococcus aureus within 24 hours of culture. Fever and culture results are summarized in Table 1. On day +5, methicillin susceptibility was confirmed, and antibiotics were changed to intravenous cefazolin plus vancomycin locks, all administered via the red lumen of the DLCVC. Following institution of this regimen, he remained afebrile with negative blood cultures. Removal of the contaminated DLCVC was deferred until after engraftment, on day +32; antibiotics were discontinued after line removal. As of this writing, he is day +117 and is doing well with no evidence of further infectious complications. Because the HSC product was infused through the same lumen that subsequently became contaminated and the susceptibility patterns of the methicillin-sensitive Staphylococcus aureus isolates from patient and HSC product were identical, PFGE was performed on both isolates.Reference Enright, Day, Davies, Peacock and Spratt 8 This analysis confirmed that the isolates were identical strains (Supplemental Figure).
TABLE 1 Timing of Fevers and Blood Culture Results by CVC Lumen
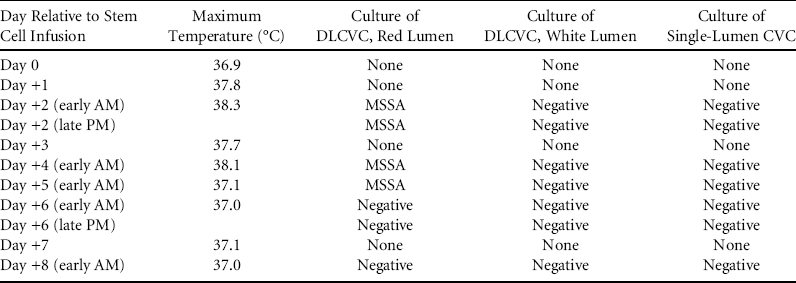
NOTE. DLCVC, double-lumen central venous catheter; CVC, central venous catheter; MSSA, methicillin-sensitive Staphylococcus aureus.
This event met the definition of a central-line–associated bloodstream infection (CLABSI) and therefore was reported to the Centers for Disease Control and Prevention (CDC) National Healthcare Safety Network. Surveillance definitions of healthcare-acquired conditions are necessarily applied uniformly. In this case, however, the investigation found convincing evidence that the infection was unavoidable. Even if graft contamination is known, it is usually impossible to withhold HSC infusion after administering the preparatory regimen. While multiple reports have found that low-grade bacterial contamination of HSC products is rarely consequential, our patient’s experience demonstrated that clinically significant infections may occur. In most reported cases, the identified contaminants are organisms of low pathogenicity such as coagulase-negative staphylococci. However, pathogenic bacteria such as E. coli and S. aureus are capable of rapid expansion within HSC products stored at room temperature.Reference Hahn, Sireis and Hourfar 9 Certain organisms, such as Candida spp and S. aureus, are particularly capable of forming biofilms on foreign material. Contamination with highly virulent organisms, while rare, may expose HCT recipients to much greater risk than contamination with less pathogenic organisms. In cases of contamination with less virulent organisms, close observation without obtaining cultures or changing antibiotic coverage may be warranted. However, in cases of contamination by virulent organisms, particularly those that tend to adhere to foreign material, a more aggressive approach may be considered. In such a case, we suggest obtaining blood cultures and considering preemptive antibiotics as guided by the identity and susceptibility of the contaminating organism. The CDC definitions of CLABSI should exempt events that are definitively unrelated to the central line.
ACKNOWLEDGMENTS
We wish to acknowledge Christa Clark, BSN, RN, CCRN, and Maria F. Gergen, MT (ASCP), of Hospital Epidemiology, University of North Carolina Hospitals, for aiding the investigation of the case. The authors report no financial support for this study. All authors report no conflicts of interest.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.285





