Diagnostic stewardship refers to the process of modifying the ordering, performing, or reporting of diagnostic tests to improve the diagnosis of and treatment of infections and other conditions.Reference Dik, Poelman, Friedrich, Niesters, Rossen and Sinha1,Reference Morgan, Malani and Diekema2 Diagnostic stewardship can be described as interventions prioritizing the right test, for the right patient, to prompt the right action. By doing so, diagnostic stewardship seeks to improve antimicrobial use, to reduce antimicrobial resistance, and to better use healthcare resources to improve patient outcomes (Fig. 1).Reference Morgan, Malani and Diekema2,Reference Dik, Poelman and Friedrich3 Historically, diagnostic stewardship was a laboratory activity that focused on optimizing specimen collection, processing, and reporting to ensure accurate test results and interpretation.Reference Rubinstein, Hirsch and Bandyopadhyay4 More recently there has been increasing understanding that test results can strongly influence antimicrobial utilization (eg, up to 80% of hospitalized patients with asymptomatic bacteriuria are inappropriately treated with antibiotics).Reference Petty, Vaughn and Flanders5 Diagnostic stewardship of microbiologic tests has evolved into a process of quality improvement utilizing multidisciplinary teams often led by healthcare epidemiology, clinical and medical microbiology, and/or antibiotic stewardship personnel.Reference Madden, Weinstein and Sifri6 Additionally, a wide array of healthcare professionals, including physicians, pharmacists, nurses, and infection preventionists, can play a role in optimizing test use and these stakeholders should be involved in the development and implementation of diagnostic stewardship initiatives.Reference Fabre, Pleiss and Klein7–Reference Patel and Fang9 Diagnostic stewardship has traditionally focused on inpatients; however, it has expanded to other healthcare settings including ambulatory and long-term care settings. The tests targeted for diagnostic stewardship vary depending on the patient population served in these different settings (eg, optimizing testing for group A Streptococcus may be a priority for ambulatory settings, whereas urine cultures and C. difficile testing may be more relevant for nursing homes).Reference Crnich, Jump, Trautner, Sloane and Mody10–Reference Mora, Krug, Grigonis, Dawson, Jing and Hammerman12

Fig. 1. Objectives of diagnostic stewardship beginning with the highest-priority objectives.
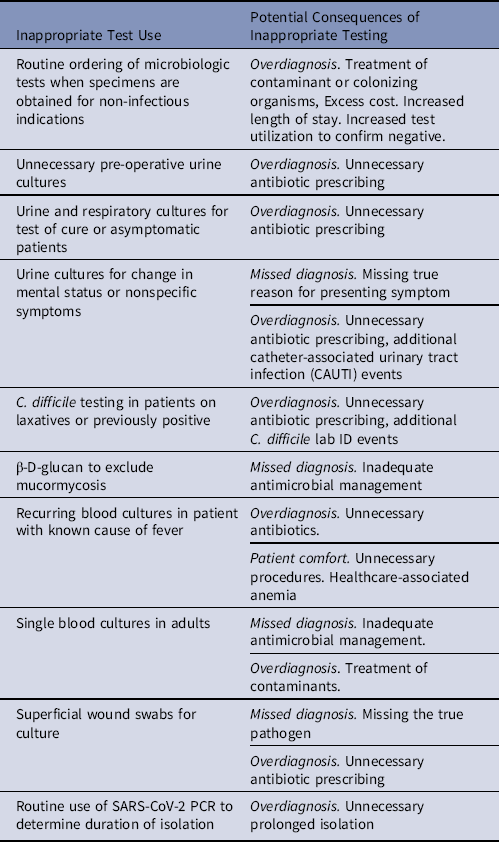
An awareness and understanding of pretest probability of infection is essential for designing diagnostic stewardship interventions that improve the usefulness of tests.Reference Sullivan13 Many diagnostic stewardship interventions function by increasing test use in high-value settings with higher probability of disease (eg, blood cultures for patients with meningitis). They also discourage or block testing in low-value settings where there is a low probability of disease and greater potential for false-positives results, which may result in patient harm (eg, blood cultures for cystitis)Reference Fabre, Sharara, Salinas, Carroll, Desai and Cosgrove14. The most common type of inappropriate microbiologic testing is overtesting, which can lead to diagnostic errors in the form of overdiagnosis, unnecessary antimicrobial treatment, and excess costReference Ganguli, Simpkin and Lupo15 A misdiagnosed infection (eg, attributing mental status changes to a UTI) can cause delays in treatment or missing the true diagnosis,Reference Filice, Drekonja, Thurn, Hamann, Masoud and Johnson16 which may have serious negative consequences for the patient (eg, mental status changes attributed to a UTI were due to a stroke). Another less frequent form of inappropriate testing is underuse of testing and potentially missed diagnoses. We provide examples of inappropriate testing and its potential clinical consequences in Table 1.
Table 1. Examples of Inappropriate Test Use That Can Be Improved Through Diagnostic Stewardship and Potential Consequences of Inappropriate Use

This document provides an overview of diagnostic stewardship with key concepts that include the multiple points in the diagnostic pathway when interventions can be implemented, the importance of multidisciplinary collaboration, and key microbiologic diagnostic tests that should be considered when building a diagnostic stewardship program. The document focuses on microbiologic laboratory testing applicable to adult and pediatric patients and is intended for a target audience that includes healthcare workers involved in developing and implementing diagnostic stewardship interventions and all workers involved in any step of the diagnostic pathway (ie, ordering, collecting, processing, reporting, and interpreting results of a diagnostic test). This document was developed by the Society for Healthcare Epidemiology of America Diagnostic Stewardship Task Force. Future work of the task force will define the relationship between diagnostic stewardship and infection prevention, antimicrobial stewardship, and pandemic management as well as needs and optimal diagnostic stewardship across patient populations (eg, immunocompromised, geriatrics) and settings (inpatient, ambulatory, emergency, and long-term care).
Process for developing this document
Leadership from the Society for Healthcare Epidemiology of America identified the need for the task force and recruited members to lead the task force (D.D. and D.M.). They then publicly called for volunteers for the Diagnostic Stewardship Task Force with the purpose of developing a series of papers better define diagnostic stewardship in healthcare settings. Experts were chosen to represent varied expertise from diverse settings. During an initial meeting, experts discussed their views on current gaps in diagnostic stewardship, and priority areas. Subsequently, subgroups were formed based on interest and area of expertise.
The diagnostic pathway
The primary objectives of diagnostic stewardship are to improve patient care by promoting accurate and timely diagnosis and thereby increasing appropriate antimicrobial use while reducing antimicrobial resistance (Fig. 1).Reference Dik, Poelman, Friedrich, Niesters, Rossen and Sinha1,Reference Morgan, Malani and Diekema2,Reference Watson, Trautner and Russo17 Additional secondary benefits of diagnostic stewardship may include improving efficiency of care, reducing costs, and improving institutional metrics related to hospital-acquired infections.Reference Madden, Weinstein and Sifri6,Reference Bhowmick, Kirn and Hetherington18,Reference Pliakos, Andreatos, Shehadeh, Ziakas and Mylonakis19
The diagnostic pathway is a process that starts and ends with clinicians who order a diagnostic test and make patient-care decisions based on their interpretation of the test result. Along the way, key steps include collection of a specimen for testing, processing the specimen, performing the test, and providing a result, all within the context of the institutional systems in place (Fig. 2). Opportunities for optimizing test use exist in each of these steps and must be carefully considered to ensure the success of a diagnostic stewardship interventions and avoid unintended consequences. We describe each step in more detail and provide a summary of diagnostic stewardship strategies and specific interventions in Tables 2 and 3, respectively.
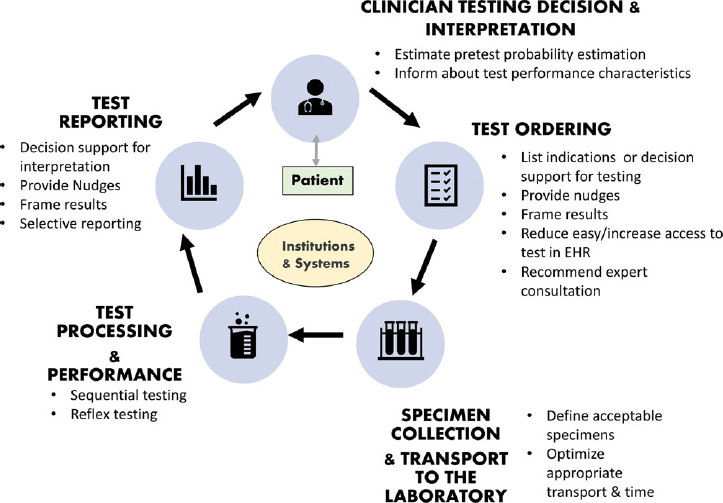
Fig. 2. Conceptual model of diagnostic stewardship and interventions at different steps in the diagnostic pathway.
Table 2. Strategies and Concepts Used in Diagnostic Stewardship
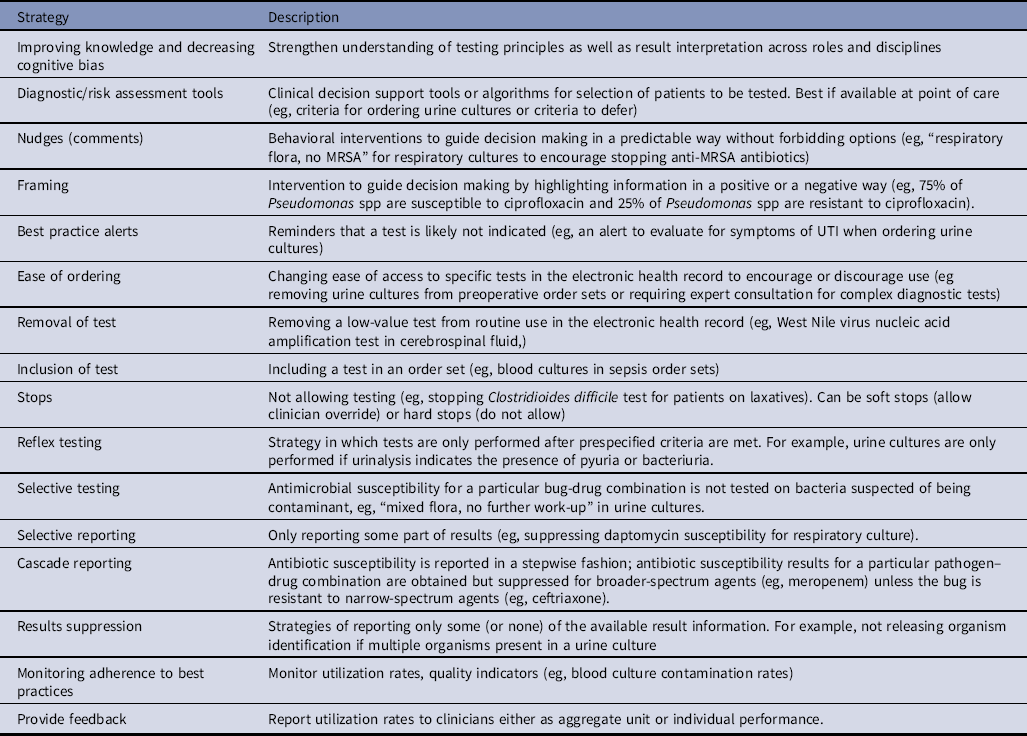
Table 3. Targets for Diagnostic Stewardship for Common Microbiologic Tests
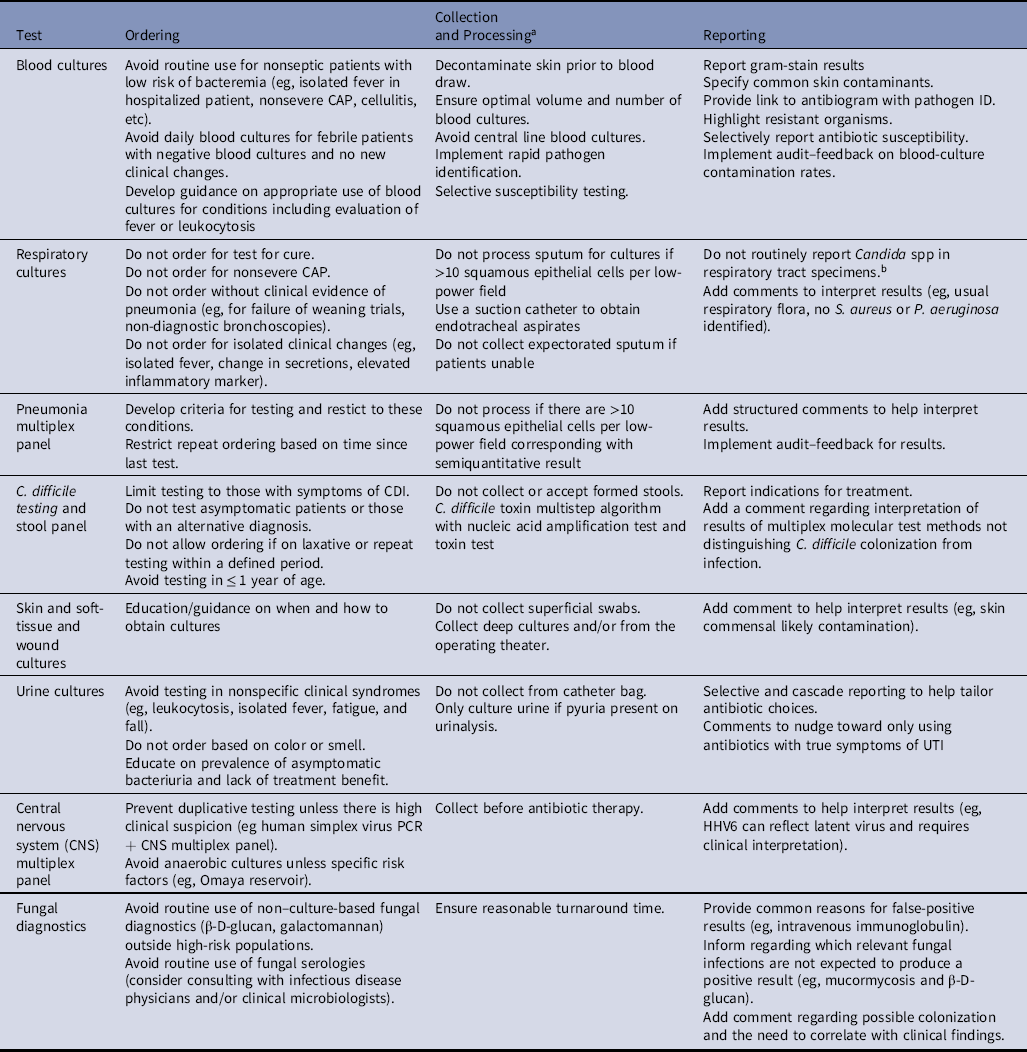
Note. PCR, polymerase chain reaction.
a Microbiology laboratories include recommendations on specimen collection and transport (eg, transport device, preservative or not, transport temperature, time from specimen collection to test) in their procedure manuals, which are available to medical personnel.
b CDC recommends reporting of Candida auris in suspected cases. C. auris screening is recommended for patients with overnight stay in a hospital outside of teh United States in the previous 1 year.
Steps in the diagnostic pathway
1. Clinician testing decisions. To ensure appropriate diagnostic test orders, clinicians must understand test performance characteristics such as sensitivity and specificity of the test and how to estimate pretest probabilities for a given patient. Pretest estimates of disease are based on (1) the incidence of disease (or base rate) in specific populations (eg, healthy vs immunocompromised patients or extremes of age) and (2) knowing how risk factors or symptoms increase or decrease the probability of disease (often expressed as likelihood ratios)Reference Sullivan13,Reference Morgan, Pineles and Owczarzak20 . This process can be particularly challenging in patient populations with nonspecific symptoms. For example, urine cultures are often part of the initial evaluation of elderly patients presenting with nonspecific symptoms such as failure to thrive or fatigue. The incidence of urinary tract infections (UTIs) in elderly women is higher than the general population, as is the prevalence of asymptomatic bacteriuria.Reference Nicolle, Gupta and Bradley21 Inappropriate ordering of urine cultures in this patient group often identifies asymptomatic bacteriuria that is subsequently inappropriately treated with antibiotics. Understanding healthcare providers’ mental constructs and local drivers for testing and management (eg, setting in which they practice, anecdotal experience, patient preferences, peer prescribing practices, etc) are essential to the design and implementation of effective diagnostic stewardship interventions.Reference Sick-Samuels, Linz and Bergmann22 Interventions focusing on clinical decision making include traditional education and various clinical-decision support tools (Table 2).
2. Test ordering. Test ordering in the electronic health record allows for numerous interventions to improve test utilization including providing informational alerts on the utility of tests for various conditions, requiring indications for test ordering, limiting test ordering based on listed indication or clinical data, and creating menus to make a test more or less orderable based on specific circumstances (eg, placing a hard stop on C. difficile orders for patients receiving laxatives). Table 2 provides a comprehensive list of diagnostic stewardship interventions and examples of interventions.
3. Specimen collection and transport to the laboratory. After the test is ordered, the sample is collected and submitted to the laboratory for processing. Inappropriate sample collection, storage, and delays in transport to the laboratory may reduce accuracy of test results.Reference Murray23 Specimen collection is performed by a wide range of individuals including phlebotomists, bedside nurses, prescribers, technicians, and even patients all with diverse levels of training. Hospitals can optimize sample collection practices by providing standard procedures and training for specimen collection. Test ordering, specimen collection, and transport to the laboratory are also referred to as the preanalytic phase of testing.
4. Test processing and performance. Within the laboratory, diagnostic stewardship includes a wide range of activities such as implementing new diagnostic methods that shorten time to appropriate therapy or new diagnostic methods have improved sensitivity to a wider range of pathogens (eg, molecular viral panel), algorithms to optimize the predictive ability of a test, such as performing sequential tests, conditional or reflexive testing (eg, only performing urine cultures when the urinalysis is abnormal), or testing only if certain criteria are met (eg, not speciating respiratory flora). Selective testing refers to the practice of not performing susceptibility testing for inappropriate situations, such as positive cultures for organisms suspected of being contaminants.24 Selective testing is an important strategy to optimize antimicrobial use. Laboratories generally employ policies that use established criteria to determine when specimens are considered contaminated and no further work-up is routinely provided (eg, 1 of 2 blood-culture sets with coagulase-negative staphylococci). This laboratory process is also known as the analytic phase of testing.
5. Test reporting. Test reporting, also referred to as the postanalytic phase of testing, can be optimized in many ways to help clinicians make more meaningful interpretations of test results and appropriate therapeutic decisions. Optimization of reporting can include comments about changes in pathogen nomenclature for easier identification of pathogens, not reporting species for organisms that are known to represent contaminants (eg, reporting yeast rather than “Candida” in respiratory cultures), statements about causes of false-negative and false-positive results, result interpretation (eg, indicating when a culture likely represents contamination), recommended therapies, recommendations for infectious disease consultation, or other explanations that complement the report.24 Although the microbiology laboratory has traditionally been responsible for test reporting, stakeholder involvement is key to ensuring that reported results lead to optimal management decisions.Reference Bhowmick, Kirn and Hetherington18,Reference Pliakos, Andreatos, Shehadeh, Ziakas and Mylonakis19,Reference Cosgrove, Li and Tamma25–Reference Musgrove, Kenney and Kendall27 For example, the antimicrobial stewardship team can offer guidance for tailored culture-result reports by prioritizing which antimicrobials are displayed based on efficacy for the disease process, formulary availability, safety, and cost as well as guidance on management (eg, dosing recommendations based on susceptibility breakpoints).Reference Musgrove, Kenney and Kendall27,Reference Langford, Seah, Chan, Downing, Johnstone and Matukas28 Detailed descriptions of culture and susceptibility reporting strategies as well as appropriate cutoffs to determine susceptibility have been published.24,Reference Langford, Leung and Haj29,30
6. Clinician test interpretation. To correctly interpret test results, clinicians must be able to assess how well a test identifies patients who have a disease and how well the test identifies those who do not have a disease.Reference Saah and Hoover31 Challenges include that available diagnostic testing for a process may differ among institutions and change over time (eg, molecular panels replacing culture-based methods). Further complicating matters, test characteristics can vary by patient population. For example, positive urine cultures have lower specificity for UTI diagnosis in the elderly, those with urinary catheters,Reference Chan-Tack, Trautner and Morgan32 or young children with bagged or clean-catch samples.Reference Etoubleau, Reveret and Brouet33,Reference Diviney and Jaswon34 Several studies have shown that clinicians have a poor understanding of test performance in various populations because as antibiotics are more likely to be prescribed based on urine and respiratory culture results than on the presence of UTI-specificReference Drekonja, Gnadt, Kuskowski and Johnson35–Reference Vaughn, Szymczak, Newton and Fakih37or pneumonia-specificReference Karsies, Tarquinio and Shein38,Reference Albin, Pogue, Petty and Kaye39 symptoms, respectively. As mentioned in the Test reporting section, several strategies can be implemented in the laboratory to support clinicians to appropriately interpret test results. Online diagnosis calculators, such as testingwisely.com, can help estimate posttest probabilities of disease. Patient-centered stewardship interventions have been successful in reducing patient or parent requests for unnecessary antibiotics.Reference Goggin, Hurley and Bradley-Ewing40,Reference Maor, Raz and Rubinstein41
Diagnostic stewardship strategies
Most diagnostic stewardship interventions focus on commonly used diagnostic tests that are major drivers of antibiotic use, such as urine or respiratory cultures.Reference Sullivan13 Other areas of attention include improving use of expensive tests that rarely influence clinical decision making (eg, respiratory virus panel for immunocompetent individuals or whole genome sequencing in the routine work-up of meningoencephalitis) and frequently obtained low-cost tests that contribute to unnecessary spending, particularly when false-positive results can lead to patient harm from additional diagnostic procedures or treatment (eg, avoiding blood cultures for isolated fever without concern for sepsis). Tests are sometimes targeted if they are used to diagnose conditions that are publicly reported, such as C. difficile tests or tests that provide indeterminate results (eg, QuantiFERON-TB Gold, β-D-glucan)Reference Fabre, Shoham, Page and Shah42,Reference Fabre, Markou and DeMallie43 or lack sensitivity and/or specificity (eg, the West Nile virus nucleic acid amplification test lacks clinical sensitivity).Reference Karaba, Blair, Martin, Saheed, Carroll and Borowitz44 At a minimum, hospitals should develop strategies for optimal practices of blood cultures, urine cultures, respiratory cultures, and C. difficile testing given the implications for patients and hospitals (Box 1).
Box 1. Reasons to Focus Diagnostic Stewardship on Blood, Urine, and Respiratory cultures, and Clostridioides difficile Testing
Blood cultures
-
One of the most commonly ordered microbiologic tests in hospitalized patients with low positivity and high risk of false-positive results (up to half of all positive blood cultures represent contaminants)
-
A significant number of blood cultures are collected as single blood cultures and/or with inappropriate blood volume.
-
Inappropriate testing may overestimate central-line–associated bloodstream infections (CLABSIs).
Urine cultures
-
One of the most common drivers of inappropriate antimicrobial use in hospitalized patients
-
Common clinical false-positive results (positive tests due to colonization without UTI)
-
Inappropriate testing may overestimate catheter-associated urinary tract infections (CAUTIs).
Respiratory cultures
-
High risk of positive results representing colonization, especially among patients with comorbidities, in the intensive care unit, or with tracheostomy
-
Common driver of inappropriate antibiotic use in hospitalized patients
C. difficile testing
-
Inappropriate testing may detect colonization and expose patients to unnecessary antibiotics.
-
Inappropriate testing may overestimate nosocomial C. difficile cases.
Diagnostic stewardship activities may rely on clinical-decision support tools, education, and testing and reporting-based strategies.Reference Morgan, Malani and Diekema2 The effectiveness of different diagnostic stewardship strategies varies widely and depend on numerous factors.Reference Mora, Krug, Grigonis, Dawson, Jing and Hammerman12,Reference Xie, Woods-Hill and King45 When considering a diagnostic stewardship intervention, one should consider not only reported effectiveness but also the feasibility of implementation based on local resources, workflow, and work preferences, as well as the burden the intervention may pose to clinicians. Some interventions are less intrusive, such as education and soft stops, but these require human resources and information technology (IT) support. The benefits of a diagnostic stewardship strategy must be carefully weighed against the potential for unintended consequences such as shifting to empiric treatment without testing. A summary of general diagnostic stewardship strategies is presented in Table 2, and a summary of potential interventions for common infectious syndromes and microbiologic tests is described in Table 3.
The impact of diagnostic stewardship interventions should be measured both for effectiveness and safety. For example, the effectiveness of an intervention to reduce inappropriate treatment of asymptomatic bacteriuria through implementation of an algorithm to reduce unnecessary urine cultures may be assessed by measuring antibiotics prescribed for “urinary tract infections” (outcome measure), the number of readmissions due to UTI or escalation of care due to missed UTI (safety measure), and frontline providers’ satisfaction with the new workflow (process measure).
More in-depth discussion of diagnostic stewardship strategies to improve antimicrobial use and hospital-acquired infection metrics and measures to evaluate the impact of diagnostic stewardship interventions will be included in a future guidance paper.
Teams and support required for diagnostic stewardship
Diagnostic stewardship efforts have been driven by a mix of motives including improving patient outcomes and antibiotic use, decreasing healthcare-associated infections, and improving cost-effectiveness. It is important that any diagnostic stewardship intervention targeting an infection be evaluated by a multidisciplinary team given the ramifications an intervention may have in terms of resources needed to carry out the intervention and the potential impact on patient management and hospital metrics (Box 2).Reference Dik, Poelman, Friedrich, Niesters, Rossen and Sinha1,Reference Patel and Fang9 For example, rapid molecular tests, which have improved pathogen detection (faster time to organism identification with identification of antimicrobial resistance through gene detection), only improved patient management in recent studies when implemented with support from an antimicrobial stewardship team that provides guidance on result interpretation and management decisions.Reference Bhowmick, Kirn and Hetherington18,Reference Cosgrove, Li and Tamma25,Reference Timbrook, Morton, McConeghy, Caffrey, Mylonakis and LaPlante26 End users should also be involved during the early stages of conception of a diagnostic stewardship intervention to ensure the proposed intervention does not increase clinical burden.
Box 2. Necessary Elements to Implement a Diagnostic Stewardship Intervention
-
1. Define a clear goal.
-
2. Ensure relevant stakeholders are involved.
-
Microbiology
-
Antimicrobial stewardship and infection control expertise
-
Information and Technology
-
Hospital leadership
-
End users (e.g. clinicians and nurses)
-
-
3. Ensure unit/hospital leadership support.
-
4. Define measures to track positive or negative impact of the intervention.
It is critical that those leading interventions have experience with clinical diagnosis and treatment as well as an understanding of the laboratory process, local processes for ordering, regulatory requirements, and quality improvement. Expertise in implementation and behavioral science is also useful to best design interventions that will change practice. Many hospitals have a laboratory utilization committee that could describe existing resources and structure to help develop and sustain diagnostic stewardship.
Many of the strategies discussed make use of the electronic medical record. IT support is important not only to “build” the intervention in the electronic medical record but also to track the impact of the intervention. Furthermore, diagnostic stewardship has not traditionally been supported financially as an independent entity in a healthcare system but as part of infection control, antimicrobial stewardship, or clinical microbiology. The time and resources required to effectively implement diagnostic stewardship changes, especially to champion the project at the unit level as well as monitor for intended and unintended consequences of interventions, can be significant, which highlights the need for support from hospital leadership. Clear leadership support can also improve frontline provider acceptance of diagnostic stewardship interventions. Many cost-saving opportunities are related to effective diagnostic stewardship, including reductions in direct costs from tests not performed and antimicrobials not prescribed and indirect costs from improved hospital-acquired infection–related metrics and reduced length of stay,Reference Watson, Trautner and Russo17,Reference Pliakos, Andreatos, Shehadeh, Ziakas and Mylonakis19,Reference Fleming, Hess and Albert46 which are relevant for making a business case to support diagnostic stewardship efforts.
Diagnostic stewardship beyond microbiology
The principles and goals of microbiology diagnostic stewardship can be applied to diagnostic tests outside microbiology, including radiology or other nonlaboratory diagnostics that affect the management of infections. Tests or evaluations that are used to diagnose infectious disease, such as chest radiographs, echocardiograms, and ophthalmologic evaluations, should be considered by diagnostic stewardship programs.
A full discussion of diagnostic stewardship for tests outside microbiology is beyond the scope of this document. Such a report would require the same approach as with infectious disease tests, including clinical evaluation by those with content expertise to evaluate the different steps in the diagnostic pathway for the test and consider potential benefits and harms to be measured.
Future needs for diagnostic stewardship
Attention to the problem of test overuse in medicine is increasing; however, the impact of inappropriate testing on patient harm has not been well studied. National management guidelines of infectious diseases often lack a diagnostic stewardship perspective.Reference Fabre, Spivak and Keller47,Reference Fabre, Carroll and Cosgrove48 Specific recommendations to improve use of common microbiologic tests, such as blood and urine cultures, have been published across age groups; however, many opportunities exist to further standardize recommendations for specific disease processes, patient populations, and testing practices.Reference Fabre, Carroll and Cosgrove48–Reference Achten, Klingenberg and Benitz54 High-risk patient populations, such as immunocompromised patients and patients living with medical devices, may also benefit from consideration of diagnostic stewardship strategies. Benchmarking has been implemented to improve patient safety in hospitals and significant efforts are ongoing to prevent healthcare-associated infections. Benchmarking of common microbiologic tests utilization does not exist but may have value as a diagnostic stewardship strategy (eg, establishing an inappropriate urine culture utilization rate for specific patient populations).Reference Fabre, Carroll and Cosgrove48,Reference Chen, Bilker, Hamilton, O’Donnell and Nachamkin55
Data on optimal measures of effectiveness, safety, and implementation are limited.Reference Mora, Krug, Grigonis, Dawson, Jing and Hammerman12 In practice, quality improvement projects are frequently implemented with a focus on test reduction and lack an evaluation of patient outcomes.Reference Mora, Krug, Grigonis, Dawson, Jing and Hammerman12 Assessment of the full impact of diagnostic stewardship interventions is needed, including potential unintended consequences for both patients and clinicians.Reference Gupta, Boland and Aron56 Lastly, the success of intervention strategies may differ depending on the implementation approach. Future studies comparing approaches and assessing implementation outcomes may identify the most efficient and effective diagnostic stewardship strategies for adoption and sustainment of practice changes.Reference Proctor, Silmere and Raghavan57
Electronic support tools have improved the use of diagnostic tests; however, there is still a great need to understand the effect of the healthcare work system and cultural and social drivers of inappropriate use of common diagnostic tests.Reference Xie, Woods-Hill and King45 Potential sociobehavioral strategies to address common mental models driving inappropriate test use have been described.Reference Vaughn, Szymczak, Newton and Fakih37
Diagnostic stewardship will need to expand to address new and expensive or unapproved tests, such as multiplex molecular panels, 16S rRNA gene sequencing, metagenomic and whole genome sequencing, to tackle both existing and emerging infectious threats such as antimicrobial resistance. Institutional support for diagnostic stewardship efforts will be important for successful implementation and sustainability of diagnostic stewardship activities and should grow beyond individual hospitals to include different healthcare settings and systems.
In summary, diagnostic stewardship is an emerging method to improve patient care by better implementing common diagnostic tests. Most changes occur in the electronic health record or laboratory and can therefore be easily scaled to large populations of patients. Comprehensive approaches to diagnostic stewardship by expert teams can reduce diagnostic errors and lead to more accurate diagnoses and treatment, reduced healthcare costs, and decreased antibiotic resistance.
Acknowledgments
We have endorsement from IDSA Diagnostics Committee, Society of Hospital Medicine, Society of Infectious Diseases Pharmacists, and Association for Professionals in Infection Control and Epidemiology. The authors thank Kristy Weinshel, SHEA Executive Director, for her assistance.
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
Dr. Van Schooneveld reports a grant and speaker fee from Biomerieux. All other authors report no conflicts of interest relevant to this article.