To the Editor—The spread of carbapenem-resistant Klebsiella pneumoniae (CR-Kp) is an emerging concern worldwide. Italy is a country endemic for Klebsiella pneumoniae carbapenemases (KPCs).Reference Papagiannitsis, Di Pilato and Giani 1 , Reference Bonura, Giuffrè and Aleo 2 KPC spread in neonatal intensive care units (NICUs) may represent a major safety issue for critical infants and a challenge in managing new admissions.Reference Giuffrè, Bonura and Geraci 3 In February 2014, we started an active surveillance program for colonization by multidrug-resistant organisms in 5 NICUs in Palermo, Italy. Inclusion criteria for patients were hospitalization for at least 48 hours and collection of at least 1 rectal swab. Samples were collected monthly in each NICU and cultured on selective media. A gram-negative bacterium resistant to at least 3 different groups of antimicrobial agents (eg, penicillins, cephalosporins, aminoglycosides, and/or carbapenems) was defined as a multidrug-resistant gram-negative (MDRGN). Colonization was defined as the isolation of an MDRGN without evidence of infection.
Swabs were inoculated onto MacConkey agar plates with 4 antibiotic disks containing gentamicin, amoxicillin-clavulanic acid, meropenem, and ceftazidime and were then incubated. Colonies growing into each antibiotic inhibition halo were subcultured, and biochemical identification of isolated strains was performed using the API 20E system (BioMerieux, Marcy-l’Etoile, France). Antibiotic susceptibility testing was performed using a disk diffusion (DD) method on Mueller-Hinton agar plates with a panel of antimicrobials (ie, netilmicin, amoxicillin-clavulanic acid, cefotaxime, ceftazidime, ceftriaxone, cefepime, imipenem, aztreonam, and gentamicin) according to the Clinical and Laboratory Standards Institute guidelines. 4 Colonies growing within meropenem and imipenem inhibition zones were subjected to E-test strips (BioMerieux, Marcy-l’Etoile, France) for minimum inhibitory concentration (MIC) determination. Antibiotic susceptibility testing and extended spectrum β-lactamase (ESBL) detection were performed using DD and a double-disk synergy test.
In December 2016, rectal swabs from 6 patients in 1 NICU identified these patients as colonized by CR-Kp, and 8 isolates were collected. Klebsiella pneumoniae strains showing an extended drug-resistant phenotype were screened by polymerase chain reaction (PCR) for the following plasmid-mediated quinolone and β-lactamase genes: qnrB, aac(6’)-Ib-cr, bla KPC, and bla CTX-M.Reference Bonura, Giuffrè and Aleo 2 Positive amplicons were sequenced to identify the resistance gene variants. Isolates were initially screened using PCR for the pilv-1 allele as a marker tracing the sequence type 258 (ST258).Reference Adler, Khabra and Chmelnitsky 5 Complete multilocus sequence typing (MLST) was then performed on all CR-Kp strains, and STs were assigned at the K. pneumoniae MLST website (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html). The first 5 cases of colonization by CR-Kp were identified on December 12, 2016. Thereafter, additional collection of rectal swabs was performed weekly until the end of the spread of the colonization cluster was proven on January 9, 2017. Colonization cluster and reported colonization cases are shown in Figure 1. All 8 CR-Kp isolates were resistant to amoxicillin-clavulanic acid, cefotaxime, ceftazidime, ceftriaxone, cefepime, imipenem, aztreonam, and gentamicin and showed an intermediate susceptibility to netilmicin. The MIC values for imipenem and meropenem were ≥16 μg/mL. The PCR results for all 8 CR-Kp isolates were positive for the bla KPC-3, bla CTX-M-15, and aac(6’)-Ib-cr genes and negative for pilv-1. Only 1 isolate (from patient 11) tested positive for qnrB. According to MLST, 7 of 8 isolates were identified as ST395, whereas the qnrB-positive isolate from patient 11 was identified as ST307. All infants included in our previous monthly surveillance activity on November 21 tested negative. All CR-Kp–colonized infants, with the exception of patient 12 (who was admitted on December 3, 2016, from another hospital outside Palermo for feeding difficulties) and patient 17, were inborn. In addition to the active surveillance program, patients 1, 12, and 17 were tested at admission with a rectal swab that showed a negative result. No microbiological data were available before December 12, 2016, to clearly identify the index patient. The simultaneous isolation of 5 CR-Kp strains was rapidly communicated to healthcare workers on the ward to control the spread to other patients. No cases of infection were reported in the colonized patients or in other infants admitted in the same weeks. The strict collaboration between laboratory staff and clinicians allowed a careful management of patients with adequate cohorting of colonized patients to avoid any restriction of new admissions to the NICU. Moreover, the molecular typing of the 8 isolates identified 1 different from the cluster, but no data were available from which to hypothesize a different source. We also performed whole-genome sequencing with a standard 2×100 PE protocol on a HiSeq 2500 instrument (Illumina, San Diego, CA)Reference Villa, Feudi and Fortini 6 and compared the genetic structures of all the isolated KPC-3-Kp ST395. For these isolates, cluster analysis based on MLST genes indicated a unique sublineage (or clonal group) of K. pneumoniae. This is not the first report of an outbreak of colonization by KPC-producing K. pneumoniae (KPC-Kp) in a NICU in Palermo; the pandemic ST258 clone has already been reported in another NICU here.Reference Giuffrè, Bonura and Geraci 3 Furthermore, in our area, the monoclonal spread of the successful pandemic ST258 clone is apparently being replaced by a simultaneous dissemination of multiple clones of KPC-Kp.Reference Geraci, Bonura and Giuffrè 7 In other recent surveillance studies from Italy,Reference Bonura, Giuffrè and Aleo 2 , Reference Richter, Franchin and Bergo 8 , Reference Gona, Barbera and Pasquariello 9 multifocal dissemination of KPC-3–producing K. pneumoniae (KPC-3-Kp) clones have been observed, showing the rapid emergence of the KPC-3-Kp ST307 clone, also coproducing the CTX-M-15 ESBL.Reference Villa, Feudi and Fortini 10 Our observation of ST395 and ST307 clones (both coproducing KPC-3 and CTX-M-15 ESBL) suggests the changing epidemiology of KPC-Kp even in specific settings such as NICUs. In conclusion, we emphasize the need for active surveillance programs focused on CR-Kp in high-risk patients and wards, such as critical infants in NICUs. Surveillance data from colonization cases could be crucial to revealing the circulation of CR-Kp in the wards, to evaluating local epidemiology, and to improving control and prevention measures.
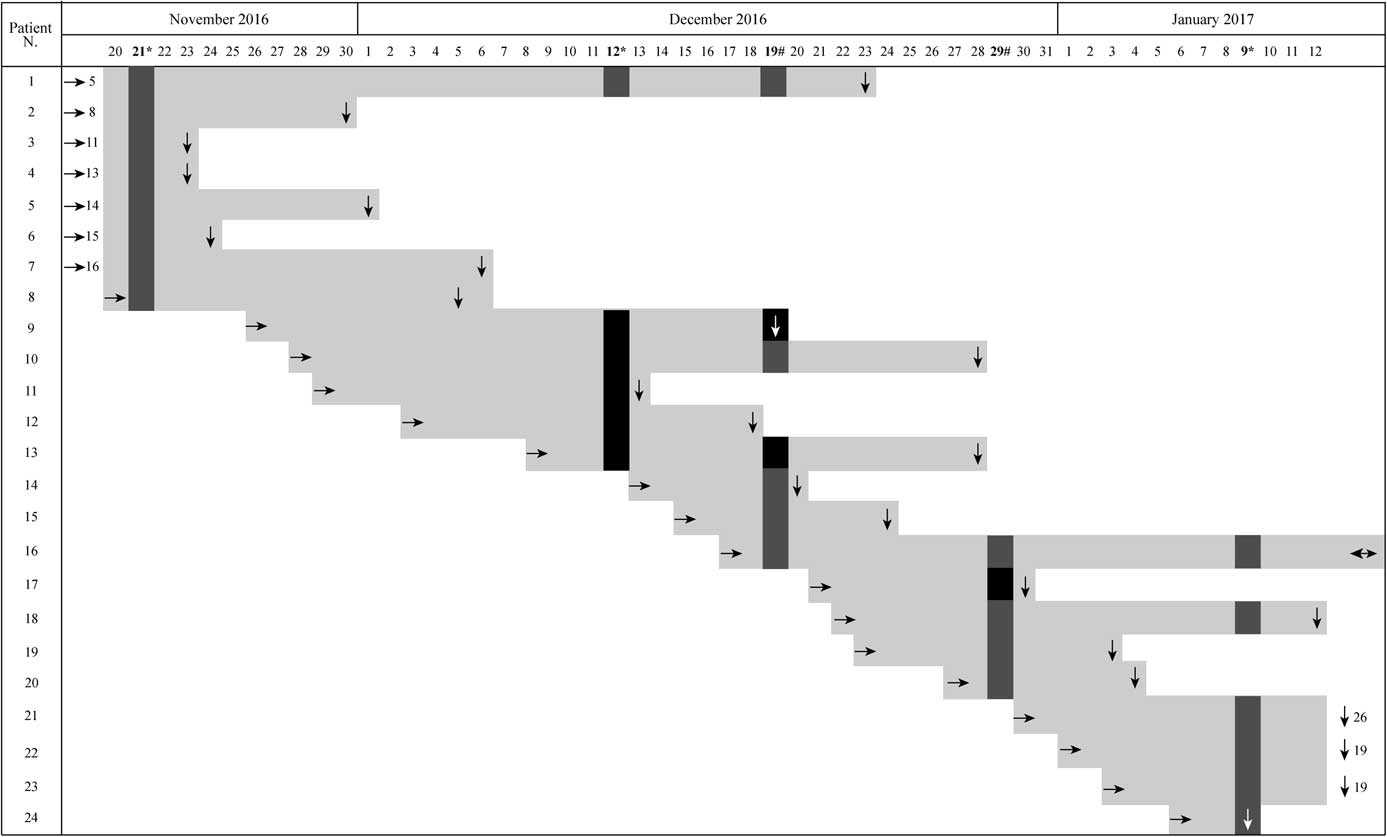
FIGURE 1 Graphical representation of positive isolation of CR-Kp with respect to admission date and hospitalization stay. Light gray bars: hospitalization stay of enrolled patients; arrows indicate: → admission date, ↓ discharge date. Sampling dates are in bold, symbols indicate: * routine sampling, # additional sampling during the outbreak. Results of the CR-Kp detection: grey boxes indicate negative samples, black boxes indicate isolation of CR-Kp.
ACKNOWLEDGMENTS
Financial support: The active surveillance program in the NICU has been funded by the Italian Ministry of Health with the Program CCM 2014.
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.



