Healthcare-associated infections (HAI) affect hundreds of millions of patients every year worldwide, resulting in prolonged length of hospital stay, long-term disability, high costs to patients and health systems, and excess deaths. 1 , 2 The causes of such infections are multifactorial. Transmission of microorganisms from a reservoir to a susceptible host plays an important part, as well as interventions that disrupt patients’ natural defenses. Within the healthcare setting, potential reservoirs include the preexisting flora of patients themselves, healthcare workers (HCWs), or the physical environment.Reference Siegel, Rhinehart, Jackson and Chiarello 3 , Reference Bonten, Hayden and Nathan 4 Contact transmission,Reference Siegel, Rhinehart, Jackson and Chiarello 3 whereby microorganisms are transmitted directly from an infected person or indirectly via a contaminated intermediate object (eg, mobile objects, medical devicesReference Schabrun and Chipchase 5 , Reference Schultsz, Meester and Kranenburg 6 ) or a person carrying transient flora,Reference Duckro, Blom, Lyle, Weinstein and Hayden 7 , Reference Pittet, Allegranzi and Sax 8 has been cited as the most common means of transferring pathogens that may result in patient colonization and infection.Reference Mayhall 9 A recent study found that infectious risk moments (IRMs), defined as seemingly innocuous yet frequently occurring care manipulations resulting in the potential transfer of pathogens to a patient, occur an average of 42.8 times per active patient care hour and 34.9, 36.8, and 56.3 times per hour in the intensive care, medical, and emergency wards, respectively.Reference Clack, Passerini, Wolfensberger, Sax and Manser 10 These findings suggest that the cumulative risk of negative patient outcomes due to IRM may be significant.
Despite growing interest to understand the role of pathogen transmission in healthcare settings, microbiological studies quantifying the risks associated with specific behaviors, such as IRMs,Reference Clack, Schmutz, Manser and Sax 11 are limited.Reference Weber and Rutala 12 , Reference Samore 13 This deficiency is perhaps due to the complexity and costs associated with the extensive environmental sampling that would be required to draw the link between behaviors and transmission dynamics. This lack of microbiological evidence likely introduces ambiguity regarding the infectious risks present during clinical care and this ambiguity may present a barrier to safe clinician behavior.
We sought expert consensus from the fields of infectious diseases, infection prevention and control (IPC), and microbiology regarding the likelihood of infectious outcomes in a series of typical care scenarios that were observed during acute care. This companion article, reported in this same issue, describes the results of structured observations to identify the frequency and nature of IRM in acute-care settings.Reference Clack, Passerini, Wolfensberger, Sax and Manser 10
We aimed to establish a comprehensive inventory of IRMs together with expert evaluations of clinical relevance. This inventory will serve the community of researchers and practitioners as a basis for designing and prioritizing future patient safety research, training, and quality improvement initiatives for infection prevention and control.
METHODS
A modified Delphi techniqueReference Hsu and Sandford 14 was used to elicit expert opinion on the likelihood of infectious outcomes (ie, patient colonization or infection) following IRMs. Experts were invited to participate in a Delphi process for an anticipated 3 rounds, or until consensus was achieved, whichever occurred first. The Delphi process was conducted in an iterative nature with subsequent rounds informed by a feedback summary of group response in the previous round whereby experts could reassess their initial responses. Surveys were distributed electronically using an online tool, allowing participates to remain anonymous and minimize conformity.Reference Hsu and Sandford 14 The Cantonal Ethics Committee of Zurich formally waived the ethics requirement for this study (KEK-StV-Nr.73/14).
Participants
We recruited a panel of international experts (nurses, physicians, and microbiologists) specialized in infectious diseases and IPC to represent a broad range of knowledge in the topic of germ transmission. We initially sent an invitation to 59 potential participants explaining the scope of the project and asking that they commit to all rounds of the Delphi process. Individuals who agreed to participate were included in the expert panel.
Survey Design
The survey consisted of 52 care scenarios that included a sample IRM observed during 130 hours of exploratory observations.Reference Clack, Passerini, Wolfensberger, Sax and Manser 10 Each IRM may be represented as a 3-part transmission pathway that identifies the surfaces (ie, source, vector, and endpoint) involved in the potential transmission of pathogens to the patient. Care scenarios were therefore selected to represent the range of observed transmission pathways based on (1) the source of pathogens, (2) the vector of transmission, and (3) the patient site (endpoint) to which the pathogens may be transferred, according to the INFORM (INFectiOus Risk Moment) structured classification taxonomy.Reference Clack, Passerini, Wolfensberger, Sax and Manser 10 We distinguished between endpoints that were noncritical sites (eg, intact skin, intact dressings, patient clothing), critical sites, defined as “body sites or medical devices that have to be protected against microorganisms potentially leading to patient infection”Reference Sax, Allegranzi, Uckay, Larson, Boyce and Pittet 15 (eg, mucous membranes, catheter insertion sites, open wounds), and patient bedding. The INFORM taxonomy excludes transmission pathways that do not end with the patient or patient bed.
The survey included 6 thematic sections based on the vectors involved: HCW hands, gloves, HCW clothing or accessories, invasive devices, medical devices, and mobile objects. The order in which the scenarios were presented within each section were block-randomized to avoid order-effect biases.Reference Perreault 16 The survey included 55 questions, 3 of which did not include scenarios meeting the current definition of IRM and are not included in this report. For each scenario, experts used a Likert-type scale to indicate the likelihood of patient colonization and patient infection, resulting in ratings for 104 items. Experts rated likelihood using the following scale: 0, none; 1, very low; 2, low; 3, medium; 4, high; or 5, very high. For all scenarios, experts were instructed to make an assessment based on an archetypical ICU patient in an 800-bed academic hospital, for which a description was provided in the survey instructions. A shortened version of the survey has previously been pilot tested.Reference Clack, Schmutz, Manser and Sax 11 Results from the pilot survey are not included in the current manuscript.
Delphi Procedure
For each Delphi round, experts received personalized access to the online survey and were instructed to complete the survey within 3 weeks. Personalized reminders were sent to all experts with partial or missing responses, 2 and 4 weeks after each initial invitation.
Round 1
Experts received access to the structured survey with all care scenarios and were instructed to judge the likelihood of (1) patient colonization and (2) patient infection for each scenario. Experts were given the opportunity to provide comments along with their ratings.
Round 2
Experts received access to the structured survey with all care scenarios, as well as a summary of round 1 results, that is, the mean ratings for likelihood of colonization and infection for each care scenario. Experts were instructed to revise their judgements or to use the comments section to specify their rational for diverging from the mean ratings.
Round 3
Experts received the structured survey including only care scenarios for which consensus had not been achieved, to reduce workload, as well as a feedback summary of round 2 results, that is, the mean ratings for likelihood of colonization and infection and the expert comments for each scenario. Experts were instructed that this was likely the final opportunity to revise their ratings and were encouraged to provide comments explaining their ratings.
Statistical Analysis
Consensus was defined a priori as 80% of participant votes falling within 2 consecutive points on a 6-point scale.Reference Ulschak 17 Statistical analyses, including measures of central tendency (means, medians, and mode), and comparison of means, were conducted using STATA version 14.2 software (StataCorp, College Station, TX) and SPSS version 23 software (IBM, Armonk, NY).
For interpretation of results, we propose a quantitative risk assessment based on Delphi expert ratings together with frequencies of IRM observed in an actual ICU.Reference Clack, Passerini, Wolfensberger, Sax and Manser 10 Each scenario from the Delphi survey was classified using the INFORM taxonomy according to the source, vector, and endpoint involved in the portrayed IRM. The frequencies during actual patient care of IRM with the same source, vector, and endpoint were extracted from Clack et alReference Clack, Passerini, Wolfensberger, Sax and Manser 10 and plotted against expert consensus ratings. By multiplying expert ratings (likelihood of colonization and likelihood of infection) for each IRM by the frequency with which that category of IRM was observed during actual care in the ICU (number of active care hours), we established a quantitative indication of the relative risk represented by each individual IRM, which we term the IRM index.
RESULTS
Following our invitation, 40 experts responded positively and formed our expert panel. The expert panel included physicians (n=30, 75%), nurses (n=71, 7.5%), and microbiologists (n=3, 7.5%), with primary specialization in infection prevention (n=22, 55%), microbiology (n=10, 25%), and infectious diseases (n=8, 20%). These participants represented the following geographic regions: Europe (n=27, 67.5%); the Americas (n=8, 20%); and the Western Pacific (n=5, 12.5%). The participation rates, despite 2 reminders in Delphi rounds 1, 2, and 3, were 92.5% (physicians 87%, nurses 86%, microbiologists 100%), 87.5% (physicians 80%, nurses 86%, microbiologists 100%), and 75% (physicians 70%, nurses 86%, microbiologists 100%) (Figure 1).
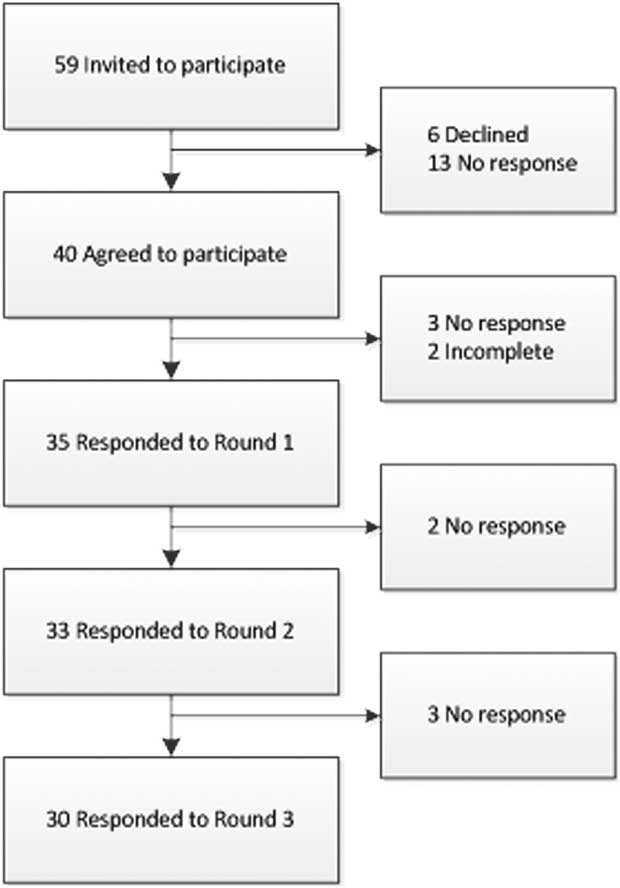
FIGURE 1 Flow chart demonstrating the participation rate of invited experts.
Following 3 Delphi rounds, consensus was achieved for 92 of 104 items (88.5%). Items for which consensus was not achieved concerned 9 colonization ratings, and 3 infection ratings and fell under the categories of invasive (n=6) and medical devices (n=2), mobile objects (n=2), HCWs (n=1), and HCW hands (n=1). We included all consensus ratings (or Delphi round 3 ratings when the prior were unavailable) as our final ratings for the analysis (Table 1). These experts did not conclude that any of the 52 scenarios represented no likelihood of colonization or infection. Expert ratings from all 3 rounds are reported in Appendix 1.
TABLE 1 Expert Consensus Ratings Grouped by VectorFootnote a

NOTE. HCW, healthcare worker.
a Expert consensus ratings are based on a Likert-type scale from 0 (none) to 5 (very high), grouped according to the vector involved in potential pathogen transfer. Groups are sorted in descending order of mean likelihood of infection. Questions within groups are sorted by descending likelihood of infection.
b Ratings for which consensus was achieved are indicated in boldface type.
The mean final ratings across all scenarios for likelihood of colonization and infection were 2.68 (95% CI, 1.73–2.02) and 2.02 (95% CI, 0.97–3.24) (Table 1). The final ratings for likelihood of colonization were higher than infection in 48 of 52 scenarios. The 4 remaining scenarios concerned moments of potential pathogen transfer to critical sites. A Wilcoxon signed-rank test determined that the increase in ratings for likelihood of colonization compared to likelihood of infection was statistically significant (z=5.92; P<.0005). Furthermore, the mean ratings across all IRM scenarios concerning potential transfer of pathogens to critical patient sites, 2.88 for colonization and 2.51 for infection, were significantly higher than for moments concerning potential transfer of pathogens to noncritical patient sites: 2.39 for colonization (P=.001) and 1.31 for infection (P<.0005). The mean ratings for likelihood of colonization and infection were grouped according to transmission vector: hands (colonization, 3.02; infection, 2.19), gloves (colonization, 2.63; infection, 2.09), HCW clothing or accessories (colonization, 2.42; infection, 1.36), invasive devices (colonization, 2.75; infection, 2.51), medical devices (colonization, 2.46; infection, 1.32), and mobile objects (colonization, 2.47; infection, 1.69). The mean ratings according to source, vector, and endpoint are shown in Figure 2.
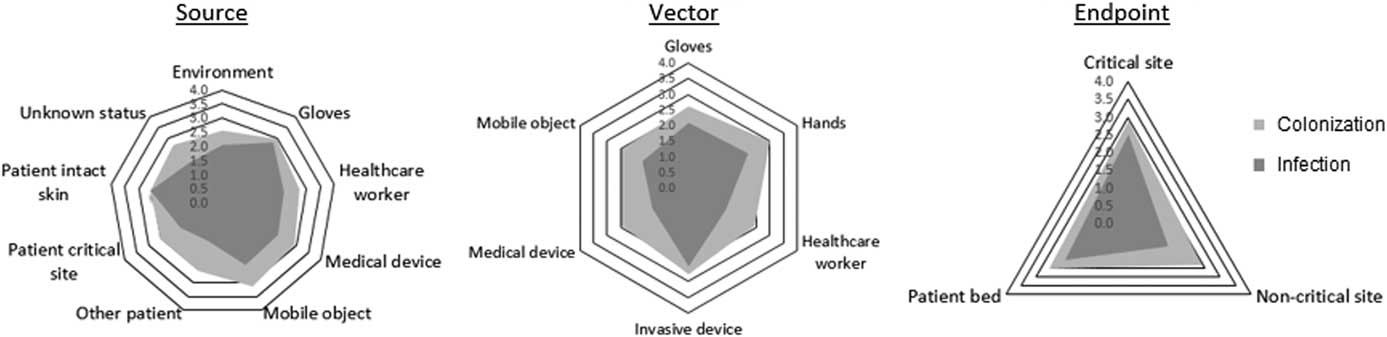
FIGURE 2 The 3 radial charts display the mean expert ratings according to the source (left), vector (middle), and endpoint (right) involved in the infectious risk moment (IRM) scenarios rated by experts. All scenarios were classified by source, vector, and endpoint according to the INFORM taxonomy.Reference Clack, Passerini, Wolfensberger, Sax and Manser 10 Ratings for colonization are shown in light grey, and ratings for infection are shown in dark grey.
The expert ratings for likelihood of colonization and infection are plotted against frequency data extracted from Clack et alReference Clack, Passerini, Wolfensberger, Sax and Manser 10 in Figure 3. The resulting relative risk indices, based on the multiplication of expert ratings and frequency of occurrence during structured observations in an ICU, are shown in Figure 4.
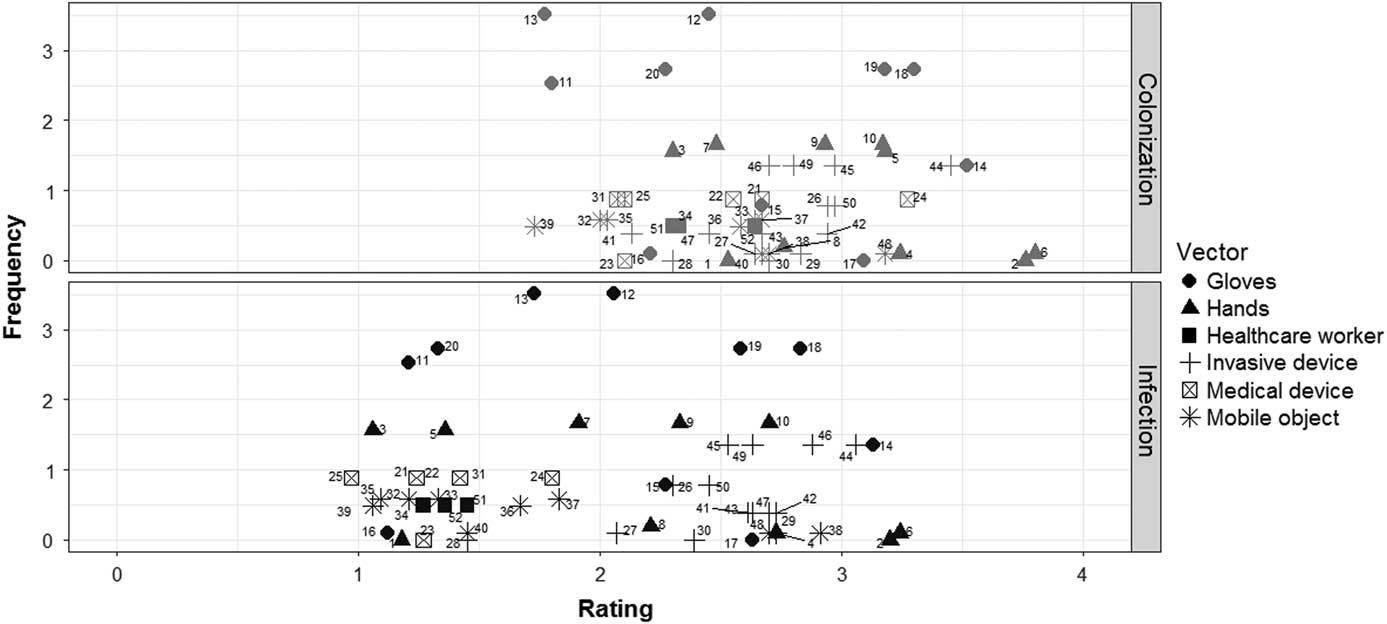
FIGURE 3 All infectious risk moments (IRMs) are plotted according to frequency of occurrence (number of IRMs per hour of active patient care) and expert rating of likelihood of infectious outcomes, colonization (marked in grey) above and infection (marked in black) below. IRMs are grouped according to the vectors involved.

FIGURE 4 The risk index for colonization (marked in grey) and infection (marked in black) of each individual infectious risk moment (IRM). The IRM index is a multiplication of the frequency with which each IRM occursReference Clack, Passerini, Wolfensberger, Sax and Manser 10 and expert ratings of likelihood of negative outcomes, colonization or infection, following the IRM.
DISCUSSION
This modified Delphi expert consensus study revealed low-medium mean ratings for the likelihood of infectious outcomes following a wide range of infectious risk scenarios observed during actual acute care. The fact that none of the 52 scenarios was rated has having no likelihood of infectious outcomes suggests that this group of experts found these IRMs to be of clinical relevance. The mean ratings for likelihood of colonization were higher than ratings for likelihood of infection, except when concerning potential pathogen transfer to critical patient body sites. Expert ratings varied particularly according to the source, vector and endpoint involved in the given scenarios (Figure 2). Although average ratings for likelihood of colonization remained relatively constant across the potential endpoints (range, 2.39–2.88), ratings for likelihood of infection were higher for scenarios concerning transfer to patient critical sites (2.51) than to the patient bed (2.08) or noncritical sites (1.31) (Figure 2). This finding is logical because the likelihood of infection is higher when pathogens are transferred directly to a critical site, where the body’s natural barrier is already broken (eg, catheter insertion site) or less resistant (eg, mucous membranes). Furthermore, although the average rating for likelihood of colonization was highest for scenarios involving hands as vectors (3.02), the average rating for likelihood of infection was highest for scenarios involving invasive devices (2.51) as vectors. Concerning the source of pathogens, average ratings for likelihood of colonization were highest among scenarios where mobile objects (3.12), gloves (2.98), and medical devices (2.92) were the sources of pathogens, whereas ratings for likelihood of infection were highest among scenarios where gloves (2.78), the patient’s own intact skin (2.59) and the healthcare worker’s own body or clothing (2.21) were the source of pathogens. This last finding is of particular interest, given that the patient’s own body may be an often-overlooked source of pathogens.
These findings are best appreciated together with the structured observations reported in our companion paper demonstrating that such IRMs occur as frequently as 34.9–56.3 times per active care hour, depending on the care setting.Reference Clack, Passerini, Wolfensberger, Sax and Manser 10 Together, these findings suggest that the cumulative risk of such IRMs on a system level may indeed present a significant threat to patient safety. The IRM index, which provides a quantitative indication of relative risk by integrating expert ratings with the frequency of individual IRMs during acute patient care,Reference Clack, Passerini, Wolfensberger, Sax and Manser 10 shows a marked and relevant variety in system level infectious risks. The IRM index, for example, demonstrates that scenarios with the highest expert ratings for likelihood of infectious outcomes did not necessarily have the highest corresponding relative risk indices due to their rare occurrence during actual patient care. Notable examples include scenarios 2, 6, and 14 (shown to the far right in Figure 3), which all include potential pathogen transfer via hands and gloves to patient critical sites, yet occur less than once per hour, resulting in relatively low risk indices (Figure 4). In contrast, scenarios with the highest relative risk indices for colonization (eg, 19 and 12) and infection (eg, 18 and 12) were those that combined medium expert ratings of infectious outcomes with high frequency, which occurred more than twice per hour of patient care.
These findings exhibit the value of our mixed-method approach, combining expert ratings with observed frequencies to provide a holistic view of infectious risks. The human-factors-informed approach of systematically identifying opportunities for transmission of pathogens also lies at the center of other landmark infection prevention strategies, such as the World Health Organization’s “Five Moments” for Hand Hygiene.Reference Pittet, Allegranzi and Sax 8 , Reference Sax, Allegranzi, Uckay, Larson, Boyce and Pittet 15 While the Five Moments model is limited to hands as the primary vector in the bidirectional exchange of microorganisms via contacts with surfaces throughout the healthcare environment, we extend this argumentation to consider the role of gloves, HCW clothing and accessories, invasive devices, medical devices, and mobile objects as vectors.
While others have noted a lack of literature documenting the risks of microbial transmission associated with HCW hands during specific care tasks,Reference Pittet, Allegranzi and Sax 8 this applies even more to the other transmission pathways addressed in our work. Thus, the Delphi technique was selected in this study to establish expert consensus considering the limited published evidence, particularly regarding the risks of patient colonization or infection associated with specific behaviors beyond hand hygiene. Specifically, using the Delphi methods over several feedback rounds has the advantage of allowing experts to exchange and reassess opinions to come to an informed consensus decision. Finally, we anticipate that the quantitative approach presented here for identifying specific behaviors associated with transmission and subsequent quantification of the likelihood of infectious outcomes may provide a basis for further quantitative modelling of system-level risks.
In the realm of healthcare safety and quality, multiple strategies have been proposed for prioritizing the behaviors addressed by improvement strategies. A critical component of this prioritization is assessing how likely the addressed behavior is to have a positive or negative impact on patient outcomes.Reference Michie, Atkins and West 18 , Reference Gurses, Murphy, Martinez, Berenholtz and Pronovost 19 Awareness of the frequency with which infectious risk behaviors occur,Reference Clack, Passerini, Wolfensberger, Sax and Manser 10 together with expert consensus regarding the likelihood of infectious outcomes, provides a basis for prioritizing the implementation of interventions that prevent the transmission of pathogens. Therefore, we introduced the IRM index (Figure 4), which considers both the likelihood of infectious outcomes at individual IRMs, as well as the frequency with which the IRM occurs during actual care, to provide a quantitative indication of relative risks on a systems level.
Ambiguity is an important barrier to healthcare worker adherence to guidelines.Reference Gurses, Seidl and Vaidya 20 We suspect that ambiguity regarding likelihood of infectious outcomes following unsafe behaviors prevents healthcare workers from developing accurate risk perceptions.Reference Sax and Clack 21 Risk perceptions play a central role in several social cognitive models as a behavioral determinant.Reference Rosenstock, Strecher and Becker 22 – Reference Bandura 24 Therefore, we believe that quantifying the risk associated with specific behaviors through expert consensus represents a first step towards removing ambiguity for healthcare workers and towards establishing informed risk perceptions to support safe behavior.
Some limitations of this study should be considered. Although 3 Delphi rounds were previously suggested as sufficient for achieving consensus,Reference Diamond, Grant and Feldman 25 we were unable to achieve consensus ratings according to our a priori definition for 12 items (11.5%). Furthermore, despite our efforts to avoid anchoring or order-effect biasesReference Sax, Allegranzi, Uckay, Larson, Boyce and Pittet 15 through block randomization of survey items, the order of blocks remained the same throughout all surveys. In addition, expert opinions may be subject to biases that may diverge from actual risks as determined through microbiology. Yet, given the current absence of the latter, expert consensus remains the most viable surrogate. Notably, despite multiple reminders, 5 experts dropped out during the Delphi process. The ratings of experts who dropped out were insignificantly lower during round 1 than experts who completed the Delphi process (data not shown). It is unlikely that this factor significantly influenced our study findings, but it should be taken into consideration when interpreting the results. Considering that all IRM scenarios examined were rated as having at least some likelihood of infectious outcome, our findings strongly support the argument to conduct more extensive microbiological studies exploring the actual transmission of microorganisms during patient care activities. Such studies should also further advance the exploration into how frequently infectious outcomes can be attributed to specific behaviors.Reference Duckro, Blom, Lyle, Weinstein and Hayden 7 , Reference Stiefel, Cadnum, Eckstein, Guerrero, Tima and Donskey 26 , Reference Ludlam, Swayne and Kearns 27
In conclusion, we believe that these findings will contribute to reducing ambiguity regarding the infectious risks associated with common clinical tasks and thus to supporting safe behavior. We further hope that establishing a comprehensive inventory of moments potentially associated with infectious outcomes, together with expert evaluations of clinical relevance, will serve the community of researchers and practitioners as a basis for prioritizing future research, training, and quality improvement initiatives.
ACKNOWLEDGMENTS
We would like to thank the following experts for their contributions to this study: Alexander Friedrich, Andie Lee, Andrea Grisold, Andreas Voss, Anita Huis, Anne Bialachowski, Bina Rubinovitch, Birgit Waitschies, Caroline Marshall, Caroline Quach, Charles Frenette, Christina Vandenbroucke-Grauls, Dale Fisher, Elisabeth Presterl, Ester Solter, Florian Salm, Heiman Wertheim, Ina Willemsen, Jan Kluytmans, Jean-Cristophe Lucet, John Ferguson, Jonas Marschall, Joost Hopman, Lindy Ryan, Margreet Vos, Mary Vearncombe, Patrice Savard, Rainer Gattringer, Rhonda Stuart, Sebastian Lemmen, Silvio Brusaferro, Simone Scheithauer, Susan Fitzgerald, Tobias Kramer, Uga Dumpis, Yehuda Carmeli, Yves Longtin.
Financial support: This study was funded by the Swiss National Science Foundation (grant no. 32003B_149474).
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.327







