The National Healthcare Safety Network (NHSN), managed by the Centers for Disease Control and Prevention (CDC), is the nation’s largest and most widely used electronic surveillance system for tracking healthcare-associated infections (HAIs). HAIs cause serious consequences for hospitalized patients, including longer duration of hospital stay and increased healthcare costs. Pediatric patients in particular are vulnerable to increased morbidity from HAIs. Understanding the epidemiology of HAIs in pediatric populations, as well as the pathogens implicated in these HAIs, can help healthcare facilities and public health agencies to develop targeted HAI prevention policies for pediatric patients.
Previous reports using NHSN data have shown that the pathogens implicated in HAIs, as well as their antimicrobial resistance patterns, vary greatly between adult and pediatric patients.Reference Hocevar, Weiner and Edwards 1 , Reference Lake, Weiner and Milstone 2 This report is the second summary of NHSN data dedicated to pathogens and antimicrobial resistance in pediatric HAIs. Federal, state, and local HAI reporting requirements enable NHSN surveillance reports to provide extensive geographic coverage of HAI data, which is nationally comprehensive for some infections. Using data from 2015–2017, this report provides information on the number, types, and common antimicrobial resistance patterns of pathogens implicated in pediatric central line–associated bloodstream infections (CLABSIs), catheter-associated urinary tract infections (CAUTIs), ventilator-associated pneumonias (VAPs, also known as pedVAPs), and surgical site infections (SSIs). These data are stratified by infection type, location within the facility, and/or surgical category. Publishing data on this granular level was made possible by the recent expansion of federal, state, and local HAI reporting requirements, and by the dedicated healthcare facilities and personnel participating in NHSN reporting.
Methods
The CLABSIs, 3 CAUTIs, 4 VAPs, 5 and SSIs 6 that occurred between 2015 and 2017 and were reported to the NHSN Patient Safety Component as of July 1, 2018, were included in this report. These data were reported by children’s hospitals or were based on pediatric patient populations within general acute-care hospitals, critical access hospitals, long-term acute-care hospitals, and inpatient rehabilitation facilities from all US states and territories. CLABSI data included events classified as mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI). Asymptomatic bacteremic urinary tract infections, CLABSIs reported from inpatient rehabilitation facilities, and outpatient SSIs were excluded from this report.
The NHSN surveillance protocols provide guidance for attributing device-associated HAIs (ie, CLABSIs, CAUTIs, VAPs) to a CDC-defined location type, and SSIs to a CDC operative procedure code. This report was limited to device-associated HAIs attributed to a neonatal or pediatric location type, and SSIs that occurred in patients <18 years old at the time of surgery. Pathogen and antimicrobial resistance data from adult locations and patients are described in a companion report.Reference Weiner-Lastinger, Abner and Edwards 7
Data from CLABSIs were stratified into 4 mutually exclusive categories based on the attributed location type: neonatal intensive care units (NICUs, level II/III and level III according to the NHSN protocol), 8 pediatric intensive care units (ICUs), pediatric oncology units (ie, pediatric oncology ICUs and wards), and pediatric wards (eg, newborn nurseries, special care nurseries, step-down units, mixed acuity units, specialty care areas, and inpatient rehabilitation units and facilities). Data from SSIs were stratified into mutually exclusive surgical categories based on the operative procedure code. Within the abdominal surgical category, pathogen distributions were also analyzed for each operative procedure code and are available in the online supplement. 9
The methods used for categorizing pathogens, reporting and interpreting antimicrobial susceptibility testing (AST) results, and defining antimicrobial resistance profiles are identical to those described in the companion report.Reference Weiner-Lastinger, Abner and Edwards 7 Up to 3 pathogens and their corresponding AST results can be reported to the NHSN for each HAI. The AST results for the drugs included in this analysis were reported using the interpretive categories of “susceptible” (S), “intermediate” (I), “resistant” (R), or “not tested.” Instead of “intermediate,” cefepime had the category interpretation of “intermediate/susceptible-dose dependent” (I/S-DD), which was considered as I for this analysis. Laboratories are expected to follow current guidelines from the Clinical and Laboratory Standards Institute (CLSI) for AST. 10 Naming conventions for pathogens used in this report generally adhered to the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) Preferred Term. 11 In some cases, pathogens were grouped by genus or clinically recognized group (eg, viridans group streptococci) (Appendices A1–A3 online). Results for Klebsiella spp were limited to K. pneumoniae and K. oxytoca; K. aerogenes was considered part of Enterobacter spp due to the timing of the NHSN’s adoption of its name change.Reference Tindall, Sutton and Garrity 12
Staphylococcus aureus was defined as methicillin-resistant (MRSA) if the isolate was reported as R to oxacillin, cefoxitin, or methicillin. Enterococcus spp isolates were defined as vancomycin-resistant (VRE) if they tested R to vancomycin. The VRE data were analyzed for all HAIs except VAP because Enterococcus spp are excluded from the NHSN VAP surveillance definition under most scenarios. Carbapenem-resistant Enterobacteriaceae (CRE) were defined as Klebsiella spp, Escherichia coli, or Enterobacter spp that tested R to imipenem, meropenem, doripenem, or ertapenem. All other pathogen–antimicrobial combinations (phenotypes) were described using a metric for nonsusceptibility, which included pathogens that tested I or R to the applicable drugs. To be defined as nonsusceptible to extended-spectrum cephalosporins (ESCs), pathogens must have tested I or R to either ceftazidime or cefepime (Pseudomonas aeruginosa) or to ceftazidime, cefepime, ceftriaxone, or cefotaxime (Klebsiella spp and E. coli). For Enterobacter spp, evaluation of nonsusceptibility to ESCs was limited to cefepime due to Enterobacter’s inducible resistance to other ESCs. Fluoroquinolone nonsusceptibility was defined as a result of I or R to either ciprofloxacin or levofloxacin (P. aeruginosa) or to ciprofloxacin, levofloxacin, or moxifloxacin (E. coli). Carbapenem nonsusceptibility in P. aeruginosa and Acinetobacter spp was defined as a result of I or R to imipenem, meropenem, or doripenem. Nonsusceptibility to aminoglycosides was defined as a result of I or R to gentamicin, amikacin, or tobramycin. Finally, multidrug-resistance (MDR) was approximated by adapting previously established definitionsReference Magiorakos, Srinivasan and Carey 13 that require nonsusceptibility to at least 1 agent within a class—thus establishing nonsusceptibility to the class—and nonsusceptibility to at least 3 of the specified classes. For Enterobacteriaceae and P. aeruginosa, 5 classes were considered in the criteria: ESCs (or cefepime for Enterobacter spp), fluoroquinolones, aminoglycosides, carbapenems, and piperacillin or piperacillin/tazobactam. A sixth class, ampicillin/sulbactam, was included in the criteria for Acinetobacter spp.
Data were analyzed using SAS version 9.4 software (SAS Institute, Cary, NC). For all HAIs and pathogens, absolute frequencies and distributions were calculated by HAI type, location type, and surgical category. The 15 most commonly reported pathogens were identified, and their frequencies and ranks within each stratum were calculated. A pooled mean percentage nonsusceptible (%NS) was calculated for each phenotype as the sum of nonsusceptible (or resistant) pathogens, divided by the sum of pathogens tested for susceptibility, and multiplied by 100. Percentage NS was not calculated for any phenotype for which <20 pathogens were tested. Differences in the %NS across location types or surgical categories were assessed for statistical significance using a mid-P exact test; pediatric wards and the abdominal surgical category were used as the referent groups for comparisons, as applicable. P < .05 was considered statistically significant. The percentage of pathogens with reported AST results (referred to as “percentage tested”) is defined elsewhereReference Lake, Weiner and Milstone 2 and was calculated for each phenotype.
Statistical analyses were not performed to test for temporal changes in the %NS during 2015–2017; thus, this report does not convey any conclusions regarding changes in antimicrobial resistance over time. Due to differences in the stratification levels, inclusion criteria, and patient populations, the %NS values in this report should not be compared with those published in previous iterations of this report.
Results
Pediatric HAI surveillance was performed by 2,545 facilities in 2015–2017, most of which were general acute-care hospitals (Table 1). In total, 18,190 pediatric HAIs and 19,978 associated pathogens were reported during this time (Table 2). Although children’s hospitals represented 4% of all facilities performing pediatric HAI surveillance, they reported 40% of all HAIs and pathogens included in this report. Staphylococcus aureus (15%), E. coli (12%), and coagulase-negative staphylococci (12%) were the 3 most commonly reported pathogens associated with all pediatric HAIs combined (Table 3).
Table 1. Characteristics of Facilities Performing Pediatric Healthcare-Associated Infection (HAI) Surveillance in the National Healthcare Safety Network, 2015–2017

a Does not include inpatient rehabilitation facilities reporting to the NHSN as locations within acute-care hospitals.
Table 2. Frequency of Pediatric Healthcare-Associated Infection (HAI) Events and Pathogens, by HAI Type, 2015–2017
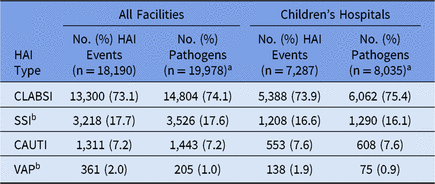
Note. CLABSI, central line-associated bloodstream infection; SSI, surgical site infection; CAUTI, catheter-associated urinary tract infection; VAP, ventilator-associated pneumonia.
a Up to 3 pathogens can be reported for each HAI event.
b SSIs and VAPs can be reported to the NHSN without an associated pathogen.
Table 3. Distribution and Rank Order of the 15 Most Commonly Reported Pathogens From All Types of Pediatric Healthcare-Associated Infections (HAIs), 2015–2017
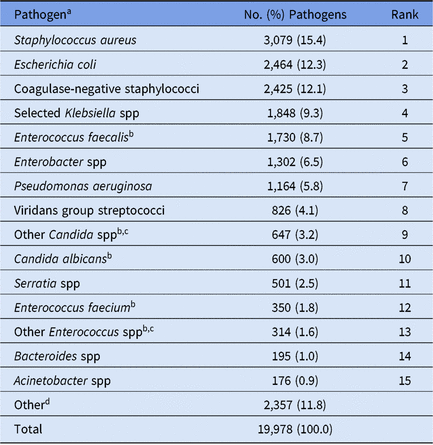
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae.
a The following species were frequently reported to the NHSN but are considered part of a larger pathogen group: Staphylococcus epidermidis (n = 1,414; 58.3% of coagulase-negative staphylococci); Enterobacter cloacae complex group (n = 1,068; 82.0% of Enterobacter spp); Serratia marcescens (n = 470; 93.8% of Serratia spp); Candida parapsilosis (n = 346; 53.5% of Other Candida spp).
b When analyzed on the genus level, Enterococcus ranks #4 and Candida ranks #6.
c The group ‘Other Enterococcus spp’ combines enterococci identified to the species level, excluding E. faecium and E. faecalis, and enterococci for which the species was not reported. The group ‘Other Candida spp’ combines Candida identified to the species level, excluding C. albicans and C. glabrata, and Candida for which the species was not reported.
d A complete distribution of all pathogens reported from pediatric HAIs can be found in the 2015–2017 Pediatric Antimicrobial Resistance Report Online Supplement (https://www.cdc.gov/nhsn/datastat/index.html).
Pathogen distributions
The highest number of CLABSI pathogens (n = 5,474; 37%) were reported from NICUs, with 740 distinct NICUs reporting at least 1 CLABSI pathogen (Table 4). Pathogens from CLABSIs were also reported in pediatric ICUs (23%), pediatric oncology units (23%), and pediatric wards (17%). The most common pathogens associated with CLABSIs varied by location type. Almost half (46%) of CLABSI pathogens from NICUs were Staphylococcus species (Table 5). Within pediatric ICUs, the most common CLABSI pathogen was E. faecalis, which was reported more frequently in pediatric ICUs than in any other pediatric location type. Viridans group streptococci and E. coli were the 2 most commonly reported CLABSI pathogens in pediatric oncology units, and Klebsiella spp (15%) and S. aureus (12%) were commonly reported from pediatric wards. Candida albicans was reported less frequently in oncology-unit CLABSIs than in CLABSIs attributed to any other location type.
Table 4. Frequency of Pediatric Device-Associated Healthcare-Associated Infection (HAI) Pathogens, by HAI and Location Type,a 2015–2017
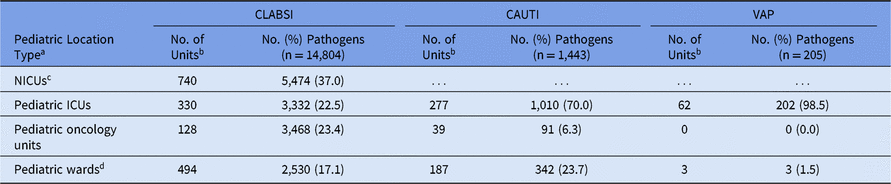
Note. CLABSI, central line-associated bloodstream infection; CAUTI, catheter-associated urinary tract infection; VAP, ventilator-associated pneumonia; NICUs, neonatal intensive care units; ICUs, intensive care units.
a Location types are mutually exclusive.
b Number of units that reported at least 1 pathogen.
c Classified by NHSN location codes as level II/III and level III NICUs. CAUTI and VAP surveillance in the NHSN are not performed in NICUs.
d Includes step-down units, mixed acuity units, and specialty care areas. Additionally, CAUTI and VAP data for ‘pediatric wards’ include events reported from free-standing inpatient rehabilitation facilities (IRFs) and rehabilitation wards located within hospitals and defined as IRFs per the Centers for Medicare and Medicaid Services (CMS).
Table 5. Distribution and Rank Ordera of the 15 Most Commonly Reported Pediatric Central Line-Associated Bloodstream Infection (CLABSI) Pathogens, by Location Type,b 2015–2017
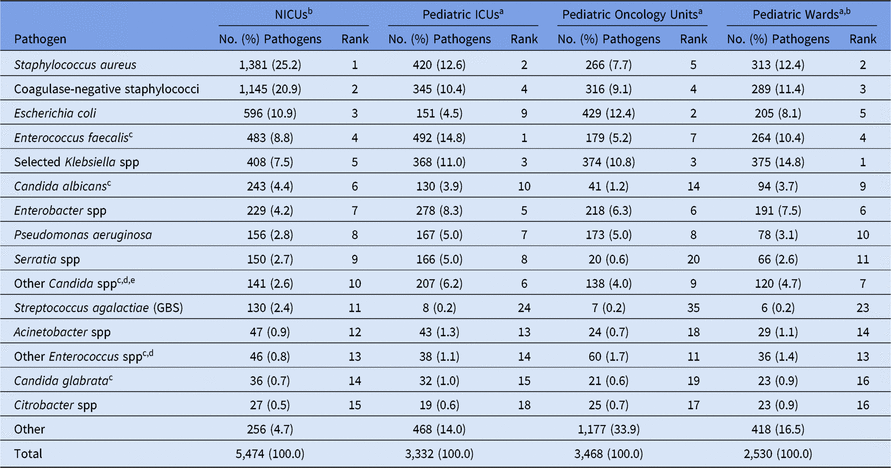
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae; NICUs, neonatal intensive care units; ICUs, intensive care units; GBS, group B streptococci.
a The 15 pathogens most commonly reported from NICUs are shown, along with their distribution and rank within the other location types. Some rankings within the other location types are not shown: ICUs: #11 (Enterococcus faecium), #12 (Stenotrophomonas maltophilia); oncology units: #1 (Viridans group streptococci; n = 507 [14.6%]), #10 (Enterococcus faecium), #11 (Rothia mucilaginosa), #13 (Stenotrophomonas maltophilia), #15 (Streptococcus pneumoniae); wards: #8 (Viridans group streptococci), #12 (Enterococcus faecium), #15 (Stenotrophomonas maltophilia).
b Location types are mutually exclusive. NICUs are classified by NHSN location codes as level II/III and level III NICUs. ‘Pediatric wards’ includes step-down units, mixed acuity units, and specialty care areas.
c When analyzed on the genus level, Candida and Enterococcus resulted in the following rankings: Candida – NICUs (#5), ICUs (#3), oncology units (#8), wards (#5); Enterococcus – NICUs (#4), ICUs (#1), oncology units (#4), wards (#2).
d The group ‘Other Enterococcus spp’ combines enterococci identified to the species level, excluding E. faecium and E. faecalis, and enterococci for which the species was not reported. The group ‘Other Candida spp’ combines Candida identified to the species level, excluding C. albicans and C. glabrata, and Candida for which the species was not reported.
e Candida parapsilosis was reported with the following frequencies: NICUs (104), ICUs (110), oncology units (55), wards (67).
Overall, 70% of CAUTI pathogens, and almost all VAP pathogens, were reported from pediatric ICUs (Table 4). Significant differences in the pathogens identified from these infections were not observed between location types; therefore, all location types were combined for the analysis of CAUTI and VAP data. Escherichia coli accounted for almost 32% of pediatric CAUTI pathogens, with P. aeruginosa and Klebsiella spp reported as the second and third most common pathogens (Table 6). Although S. aureus (23%) and P. aeruginosa (16%) were the 2 most commonly reported VAP pathogens and were frequently reported for other HAI types, Serratia spp (8%), Haemophilus influenzae (7%), and Stenotrophomonas maltophilia (7%) were commonly reported only for VAPs (Table 7).
Table 6. Distribution and Rank Order of the 15 Most Commonly Reported Pediatric Catheter-Associated Urinary Tract Infection (CAUTI) Pathogens, 2015–2017
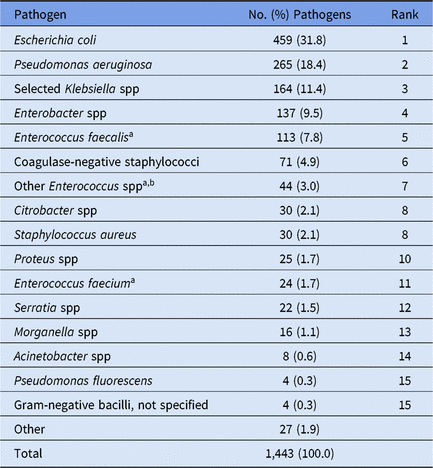
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae.
a When analyzed on the genus level, Enterococcus ranks #3.
b The group ‘Other Enterococcus spp’ combines enterococci identified to the species level, excluding E. faecium and E. faecalis, and enterococci for which the species was not reported.
Table 7. Distribution and Rank Order of the 15 Most Commonly Reported Pediatric Ventilator-Associated Pneumonia (VAP) Pathogens, 2015–2017
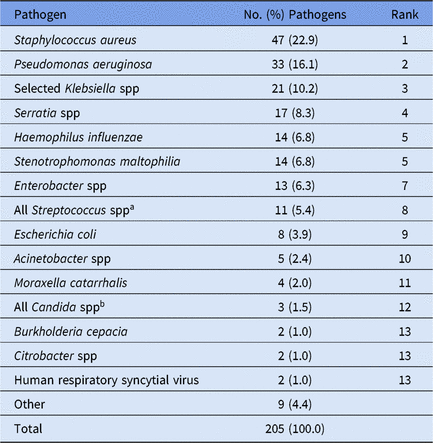
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae.
a The group 'All Streptococcus spp’ aggregates all members of genus Streptococcus, including those not identified to the species level.
b The group ‘Other Candida spp’ combines Candida identified to the species level, excluding C. albicans and C. glabrata, and Candida for which the species was not reported. Candida can be reported to the NHSN as pediatric VAP pathogens when identified in lung tissue or pleural fluid, or in an immunocompromised patient when a matching Candida organism is identified from blood and a lower respiratory tract specimen. More information is available in the NHSN Patient Safety Component Protocol.
Most pediatric SSI pathogens (63%) were reported following abdominal surgeries, and more than half of these occurred in organ/space infections (Table 8). The type of SSI and pathogen varied by surgical procedure category; half of orthopedic pathogens were identified in deep incisional infections, almost 78% of neurosurgical pathogens were identified as organ/space infections, and cardiac and obstetrical/gynecological pathogens were commonly identified in superficial incisional infections. Staphylococcus aureus was the most common pathogen for orthopedic, neurosurgical, and cardiac SSIs, but E. coli was reported more frequently in abdominal SSIs than in any other surgical category reviewed (Table 9). Coagulase-negative staphylococci were particularly common among SSIs following neurosurgical procedures (21%), second only to S. aureus (28%).
Table 8. Frequency and Types of Pediatric Surgical Site Infection (SSI) Pathogens, by Surgical Category, 2015–2017
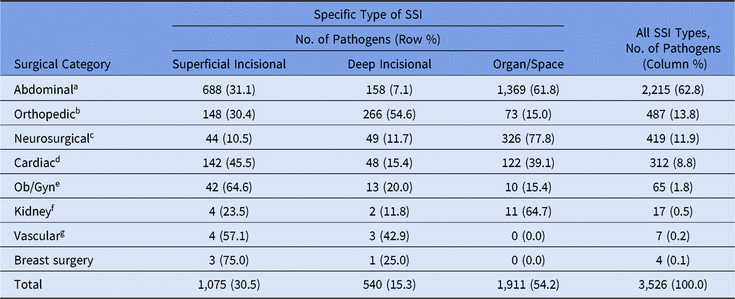
Note. Ob/Gyn, obstetrical and gynecological.
a Appendix surgery, bile duct, liver, or pancreatic surgery, liver transplant, gallbladder surgery, colon surgery, gastric surgery, herniorrhaphy, small bowel surgery, spleen surgery, exploratory laparotomy, and rectal surgery.
b Open reduction of fracture, hip prosthesis, knee prosthesis, limb amputation, spinal fusion/refusion, and laminectomy.
c Craniotomy and ventricular shunt.
d Cardiac surgery, heart transplant, coronary artery bypass graft with chest incision with or without donor incision, pacemaker surgery, and thoracic surgery.
e Cesarean section, abdominal hysterectomy, ovarian surgery, and vaginal hysterectomy.
f Kidney surgery and kidney transplant.
g Abdominal aortic aneurysm repair, shunt for dialysis, carotid endarterectomy, and peripheral vascular bypass surgery.
Table 9. Distribution and Rank Order of the 15 Most Commonly Reported Pediatric Surgical Site Infection (SSI) Pathogens, by Surgical Category,a 2015–2017
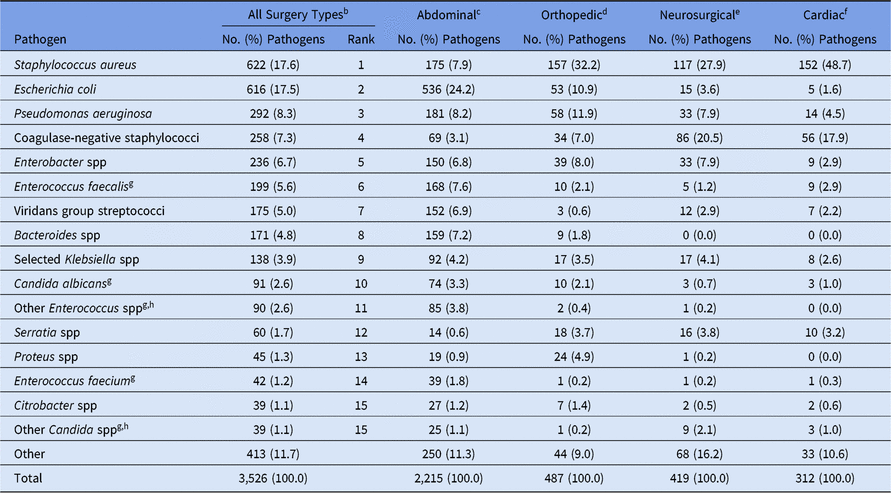
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae.
a The 4 most commonly reported surgical categories by pathogen volume are shown.
b Consists of all NHSN pediatric operative procedure codes and is not limited to the 4 surgical categories shown in the table.
c Appendix surgery, bile duct, liver, or pancreatic surgery, liver transplant, gallbladder surgery, colon surgery, gastric surgery, herniorrhaphy, small bowel surgery, spleen surgery, exploratory laparotomy, and rectal surgery. The 2015–2017 Pediatric Antimicrobial Resistance Report Online Supplement contains pathogen distributions stratified by individual NHSN procedure code within the abdominal surgical category (https://www.cdc.gov/nhsn/datastat/index.html).
d Open reduction of fracture, hip prosthesis, knee prosthesis, limb amputation, spinal fusion/refusion, and laminectomy.
e Craniotomy and ventricular shunt.
f Cardiac surgery, heart transplant, coronary artery bypass graft with chest incision with or without donor incision, pacemaker surgery, and thoracic surgery.
g When analyzed on the genus level, across all surgery types, Enterococcus ranks #3 and Candida ranks #9.
h The group ‘Other Enterococcus spp’ combines enterococci identified to the species level, excluding E. faecium and E. faecalis, and enterococci for which the species was not reported. The group ‘Other Candida spp’ combines Candida identified to the species level, excluding C. albicans and C. glabrata, and Candida for which the species was not reported.
Percentage tested and percentage nonsusceptibility
Among pediatric CLABSIs, although AST results were reported for most gram-positive pathogens, <85% of many of the gram-negative pathogens were reported with selected susceptibility results (Table 10). The percentage of pediatric CLABSI pathogens with nonsusceptibility varied by location type. The NICUs had the lowest %NS values for almost all phenotypes. For example, ESC nonsusceptibility in Klebsiella spp, E. coli, and P. aeruginosa, MDR E. coli and Enterobacter spp, and fluoroquinolone nonsusceptibility in P. aeruginosa were all statistically significantly lower in NICUs than in pediatric wards (eg, 8% of E. coli in NICUs were nonsusceptible to ESCs, compared with 23% in pediatric wards). Significant differences in CLABSI pathogen nonsusceptibility were not observed between pediatric ICUs and pediatric wards, except for carbapenem resistance among Klebsiella spp, which was significantly more prevalent in ICUs (3%) than in wards (1%). Pediatric oncology units tended to have a higher %NS compared with pediatric wards, such as E. faecium resistance to vancomycin (55% in oncology units vs 33% in wards).
Table 10. Percentage of Pathogens Reported from Pediatric Central Line-Associated Bloodstream Infections (CLABSIs) that Tested Nonsusceptiblea (NS) to Selected Antimicrobial Agents by Location Type,b 2015–2017
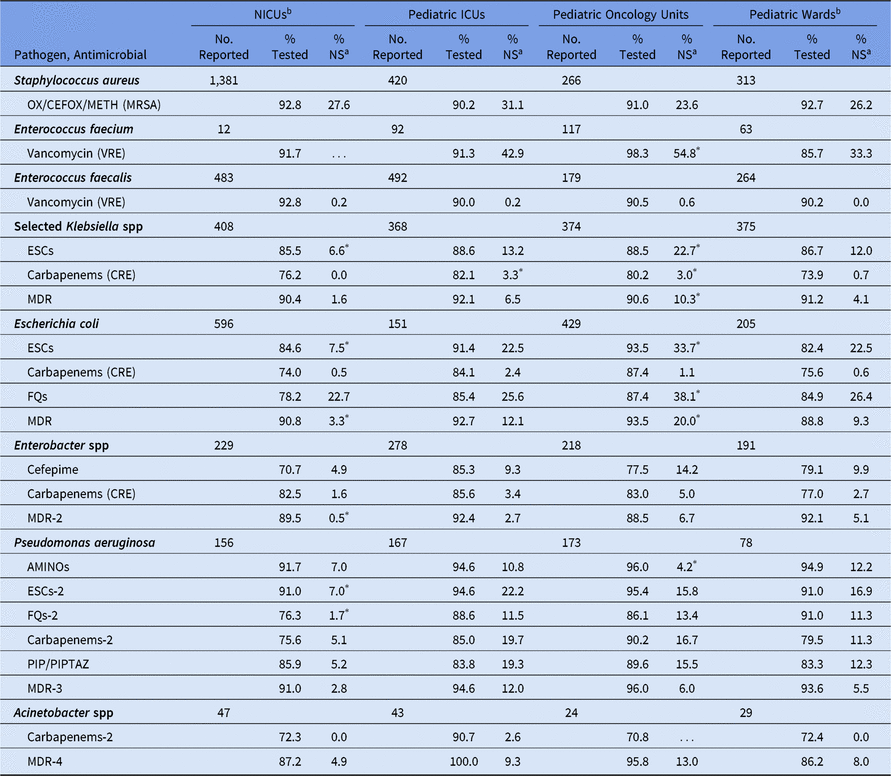
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae; NICUs, neonatal intensive care units; ICUs, intensive care units; OX/CEFOX/METH, oxacillin, cefoxitin, or methicillin; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ESCs, extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, or ceftriaxone); CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); MDR, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs, FQs, AMINOs, carbapenems (R only), PIPTAZ); MDR-2, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: cefepime, FQs, AMINOs, carbapenems (R only), PIPTAZ); FQs, fluoroquinolones (ciprofloxacin, levofloxacin, or moxifloxacin); AMINOs, aminoglycosides (amikacin, gentamicin, or tobramycin); ESCs-2, extended-spectrum cephalosporins (cefepime or ceftazidime); FQs-2, fluoroquinolones (ciprofloxacin or levofloxacin); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam; Carbapenems-2, imipenem, meropenem, or doripenem; MDR-3, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ); MDR-4, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ, ampicillin/sulbactam).
* Statistically significantly different than %NS in pediatric wards; P < .05.
a MRSA, VRE, and CRE data are presented as %R (ie, includes only those pathogens that tested resistant). All other phenotypes are shown as %NS (ie, includes pathogens that tested intermediate or resistant). This metric is only calculated when at least 20 isolates have been tested.
b Location types are mutually exclusive. NICUs are classified by NHSN location codes as level II/III and level III NICUs. ‘Pediatric wards’ includes step-down units, mixed acuity units, and specialty care areas.
For CAUTIs, the %NS did not vary by location type. We noted 2 exceptions: E. coli reported from pediatric wards had a higher %NS to fluoroquinolones (21%) compared with those reported from pediatric ICUs (18%), and had a statistically significantly higher percentage resistant to carbapenems (3%) compared with pediatric ICUs (0%) (data not shown). Overall, 21% of CAUTI E. coli were nonsusceptible to ESCs, and <1% were resistant to carbapenems (Table 11). Most VAP pathogen phenotypes had <20 pathogens tested; therefore, %NS values were not calculated.
Table 11. Percentage of Pathogens Reported from Pediatric Catheter-Associated Urinary Tract Infections (CAUTIs) and Ventilator-Associated Pneumonias (VAPs) that Tested Nonsusceptiblea (NS) to Selected Antimicrobial Agents, 2015–2017
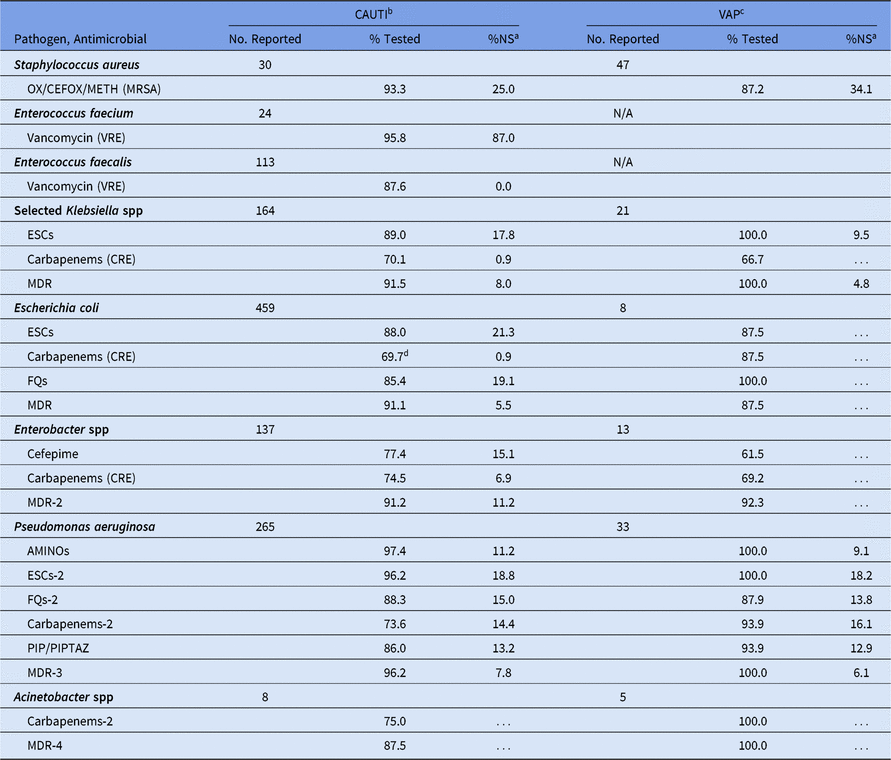
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae; OX/CEFOX/METH, oxacillin, cefoxitin, or methicillin; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ESCs, extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, or ceftriaxone); CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); MDR, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs, FQs, AMINOs, carbapenems (R only), PIPTAZ); MDR-2, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: cefepime, FQs, AMINOs, carbapenems (R only), PIPTAZ); FQs, fluoroquinolones (ciprofloxacin, levofloxacin, or moxifloxacin); AMINOs, aminoglycosides (amikacin, gentamicin, or tobramycin); ESCs-2, extended-spectrum cephalosporins (cefepime or ceftazidime); FQs-2, fluoroquinolones (ciprofloxacin or levofloxacin); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam; Carbapenems-2, imipenem, meropenem, or doripenem; MDR-3, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ); MDR-4, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ, ampicillin/sulbactam).
a MRSA, VRE, and CRE data are presented as %R (ie, includes only those pathogens that tested resistant). All other phenotypes are shown as %NS (ie, includes pathogens that tested intermediate or resistant). This metric is only calculated when at least 20 isolates have been tested.
b Almost no statistically significant differences in CAUTI %NS were observed across pediatric location types. Therefore, all locations have been combined.
c ~99% of pediatric VAP pathogens were reported from a pediatric intensive care unit. VRE data are not shown for VAP because Enterococcus spp are typically excluded from VAP surveillance.
d If the percentage tested is <70%, caution should be used when interpreting the %NS.
Among SSIs, the %NS was calculated for all surgical categories combined, as well as for the 4 surgical categories with the highest pathogen volume. Orthopedic SSIs had a significantly higher %NS for ESC and fluoroquinolone nonsusceptible E. coli, and fluoroquinolone nonsusceptible P. aeruginosa, compared with abdominal SSIs (Table 12). However, the percentage of S. aureus resistant to methicillin was significantly lower in orthopedic SSIs (19%) compared with abdominal SSIs (34%). Significant differences in %NS were not observed when comparing abdominal SSIs to neurosurgical SSIs, and too few pathogens were reported from cardiac SSIs to calculate a %NS for most phenotypes.
Table 12. Percentage of Pathogens Reported from Pediatric Surgical Site Infections (SSIs) that Tested Nonsusceptiblea (NS) to Selected Antimicrobial Agents, by Surgical Categoryb, 2015–2017
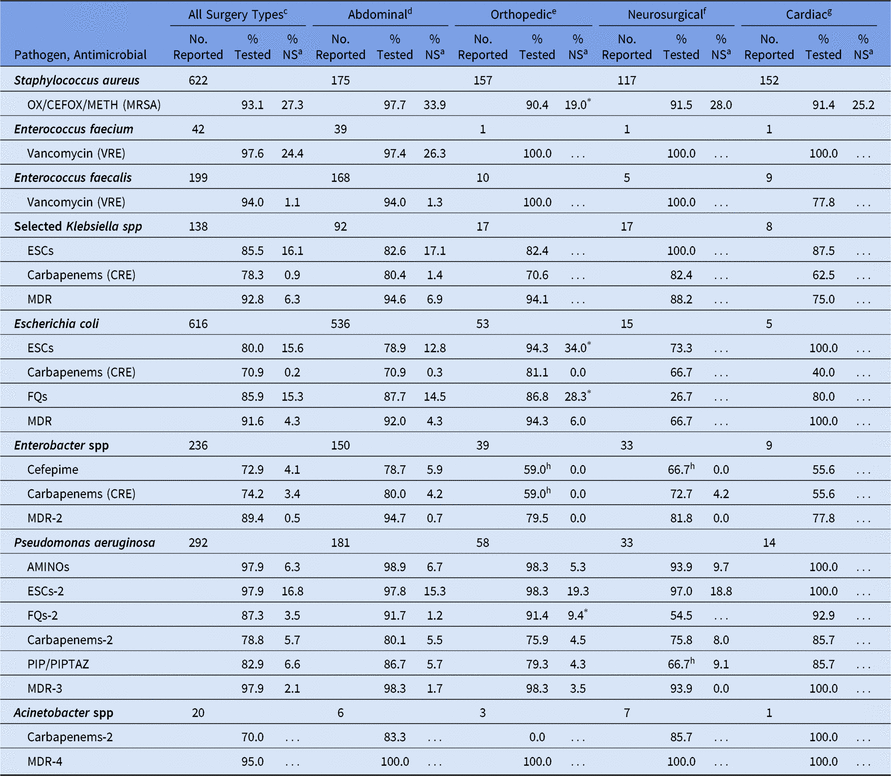
Note. Selected Klebsiella spp, K. oxytoca and K. pneumoniae; OX/CEFOX/METH, oxacillin, cefoxitin, or methicillin; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant Enterococcus; ESCs, extended-spectrum cephalosporins (cefepime, cefotaxime, ceftazidime, or ceftriaxone); CRE, carbapenem-resistant Enterobacteriaceae (imipenem, meropenem, doripenem, or ertapenem); MDR, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs, FQs, AMINOs, carbapenems (R only), PIPTAZ); MDR-2, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: cefepime, FQs, AMINOs, carbapenems (R only), PIPTAZ); FQs, fluoroquinolones (ciprofloxacin, levofloxacin, or moxifloxacin); AMINOs, aminoglycosides (amikacin, gentamicin, or tobramycin); ESCs-2, extended-spectrum cephalosporins (cefepime or ceftazidime); FQs-2, fluoroquinolones (ciprofloxacin or levofloxacin); PIP, piperacillin; PIPTAZ, piperacillin/tazobactam; Carbapenems-2, imipenem, meropenem, or doripenem; MDR-3, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ); MDR-4, multidrug-resistant (NS to 1 drug in at least 3 of the following classes: ESCs-2, FQs-2, AMINOs, carbapenems-2, PIP/PIPTAZ, ampicillin/sulbactam).
* Statistically significantly different than %NS among abdominal SSIs; P < .05.
a MRSA, VRE, and CRE data are presented as %R (ie, includes only those pathogens that tested resistant). All other phenotypes are shown as %NS (ie, includes pathogens that tested intermediate or resistant). This metric is only calculated when at least 20 isolates have been tested.
b The 4 most commonly reported surgical categories by pathogen volume are shown.
c Consists of all NHSN pediatric operative procedure codes and is not limited to the 4 surgical categories shown in the table.
d Appendix surgery, bile duct, liver, or pancreatic surgery, liver transplant, gallbladder surgery, colon surgery, gastric surgery, herniorrhaphy, small bowel surgery, spleen surgery, exploratory laparotomy, and rectal surgery.
e Open reduction of fracture, hip prosthesis, knee prosthesis, limb amputation, spinal fusion/refusion, and laminectomy.
f Craniotomy and ventricular shunt.
g Cardiac surgery, heart transplant, coronary artery bypass graft with chest incision with or without donor incision, pacemaker surgery, and thoracic surgery.
h If the percentage tested is <70%, caution should be used when interpreting the %NS.
Discussion
Previous reports on pediatric HAIs are sparse, with many studies restricted to a few hospitals or a limited time period.Reference Zhang, Xu and Langley 14 – Reference Simon, Ammann and Bode 16 While many studies provide data on HAI incidence among pediatric patients, few focus on pathogens and antimicrobial resistance profiles. This report is an update of the NHSN’s previous pediatric-specific HAI antimicrobial resistance report,Reference Lake, Weiner and Milstone 2 and it provides a summary of pathogens and AST data reported from >2,500 healthcare facilities. This report can be used by the pediatric infectious disease community and other pediatric or neonatal healthcare professionals, as well as by infection control and public health organizations, to inform prevention and antimicrobial stewardship policies that seek improvements in antimicrobial prescribing for pediatric patients.
Overall, 60% of pathogens included in this analysis were reported from general acute-care hospitals. Because of federal reporting requirements for participation in the Centers for Medicare and Medicaid Services (CMS) Quality Reporting Programs (QRPs), 17 – 19 nearly all general acute-care hospitals in the United States use the NHSN to identify and report HAIs. The CMS Hospital Inpatient QRP requires general acute-care hospitals to report CLABSIs and CAUTIs to the NHSN that occur in adult, pediatric, and neonatal ICUs, and in 2015, this requirement was extended to adult and pediatric medical, surgical, and medical/surgical wards. Compared with the prior report,Reference Lake, Weiner and Milstone 2 a larger proportion of CLABSI and CAUTI pathogens in this analysis were reported from pediatric wards due to the expanded reporting requirements. In addition, acute-care hospitals participating in the Hospital Inpatient QRP are required to perform surveillance for select SSIs. Any SSI occurring in a pediatric patient following a colon surgery is reported to the NHSN. Pediatric VAPs are not required to be reported under any CMS QRP; thus, they are reported to the NHSN either voluntarily or in response to a state or local reporting mandate.
Approximately 40% of pathogens in this analysis were reported from designated children’s hospitals. Although children’s hospitals are not included in any CMS QRP and are not federally required to report HAI data to the NHSN, 92 children’s hospitals were performing HAI surveillance in the NHSN between 2015 and 2017, representing almost all children’s hospitals in the nation. Overall, due to the NHSN’s scope of data collection and the magnitude of facilities participating in pediatric HAI surveillance, this report offers the most comprehensive summary available of pediatric HAI pathogens and their antimicrobial resistance profiles.
The CLABSI pathogens varied by location type and were particularly distinctive among infections reported from pediatric oncology units. Viridans group streptococci and E. coli were the 2 most commonly reported CLABSI pathogens in pediatric oncology units, but in accordance with previous findings, S. aureus and/or coagulase-negative staphylococci were commonly reported from NICUs and pediatric ICUs.Reference Lake, Weiner and Milstone 2 , Reference Rutledge-Taylor, Matlow and Gravel 15 Viridans group streptococci and E. coli were also common CLABSI pathogens in adult oncology units and can be attributed to mucosal barrier injury frequently experienced by oncology patients exposed to cytotoxic chemotherapy.Reference Weiner-Lastinger, Abner and Edwards 7 , Reference See, Freifeld and Magill 20 In 2015–2017, the 3 most commonly reported pediatric CAUTI and VAP pathogens remained consistent with the results from 2011–2014Reference Lake, Weiner and Milstone 2 ; E. coli accounted for almost 32% of CAUTI pathogens and S. aureus accounted for 23% of VAP pathogens.
In general, the pathogens associated with pediatric HAIs were less likely to have antimicrobial resistance phenotypes than pathogens from adult HAIs.Reference Weiner-Lastinger, Abner and Edwards 7 This finding may be due to pediatric patients’ lower exposures to healthcare settings and antimicrobials than adult patients over time.Reference Lake, Weiner and Milstone 2 Within pediatric CLABSI data, the %NS varied by location type with NICU pathogens having a significantly lower %NS, and pediatric oncology pathogens having a higher %NS, than most pathogens from pediatric wards. Particularly for most CLABSI E. coli and Klebsiella spp phenotypes, nonsusceptibility was significantly higher in pediatric oncology units than general pediatric wards (eg, 20% of E. coli were MDR in oncology units, compared with 9% in pediatric wards). The opposite finding was seen among adult CLABSI data, in which adult oncology units tended to have a lower %NS for most Klebsiella spp, P. aeruginosa, and Acinetobacter spp phenotypes compared with adult wards.Reference Weiner-Lastinger, Abner and Edwards 7 Although it is not clear why these findings would differ between adult and pediatric settings, differences in underlying illnesses, healthcare exposures, and overall antimicrobial exposures may be explanatory factors.
The sparsity of data reported to the NHSN for some CAUTI and most VAP resistance profiles limits the extent to which these data can be analyzed and interpreted. Select pathogens of interest were rarely identified in SSIs following neurosurgical and cardiac procedures, and in many cases, we were not able to calculate a %NS. Also, ESC nonsusceptibility in E. coli was notably high among pediatric orthopedic SSIs (34%). This percentage was higher than the %NS for all surgical categories combined (16%), and higher than the corresponding %NS among adult patients (17%).Reference Weiner-Lastinger, Abner and Edwards 7 The total number of E. coli reported from pediatric orthopedic SSIs was low (53 E.coli pathogens reported). Thus, the high %NS could be the result of low precision in the data, or may be related to differences between pediatric patients undergoing orthopedic procedures compared with other surgery types and compared with adult patients undergoing orthopedic procedures.
Our report has several limitations. All NHSN data are self-reported, and the validation of HAI, pathogen, and AST results is limited to what healthcare facilities, state health departments, and CMS validate. Typically, pathogen and AST results are not the focus of these validation efforts. This report applies only to NHSN-defined HAIs and does not account for any community-acquired infections or antimicrobial resistance that occurs outside of an inpatient healthcare facility. Differences may exist between facilities or laboratories in their testing practices, reporting methods, and breakpoint interpretations, which cannot be accounted for in this analysis. In addition, the low volume of pediatric pathogens reported for certain location types or surgical categories, and the low percentage of gram-negative pathogens reported with AST results, make the interpretation of these data difficult. When the percentage of pathogens tested for susceptibility is <70%, caution should be used when interpreting the resulting %NS. Furthermore, the phenomenon of “selective reporting” can cause some pathogens to be reported to the NHSN as “not tested” to certain drugs due to suppression rules in place as part of antimicrobial stewardship efforts. Although this may have resulted in a slight underestimation of the national percentage tested and overestimation of the national %NS, the impact of selective reporting has been thoroughly assessed in a separate report and was deemed to have a minimal impact on national data.Reference Weiner-Lastinger, Abner and Edwards 7
In conclusion, national data on the epidemiology of resistant pathogens associated with pediatric HAIs provide a useful resource for the pediatric healthcare community that can aid in target setting by antimicrobial stewardship programs and provide guidance for HAI prevention efforts. Given the differences in these data compared with adult HAIs, facilities are encouraged to review their local pathogen and antimicrobial resistance data specific to pediatric patients, which can help inform interventions to prevent the spread of resistant organisms.
Acknowledgments
We thank NHSN-participating healthcare facilities and the infection control community for their diligent efforts to monitor infections, to reduce antimicrobial resistance, and to improve patient safety. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Diseases Registry.
Financial support
The NHSN surveillance system is supported by the Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention.
Conflicts of interest
All other authors report no conflicts of interest relevant to this article.
Appendices
A1. Organisms Included in NHSN’s ‘Enterobacter spp’ Group for this Report
A2. Organisms Included in NHSN’s ‘Coagulase-Negative Staphylococci’ Group for this Report
A3. Organisms Included in NHSN’s ‘Viridans Group Streptococci’ Group for this Report