To the Editor—Preauthorization is a fundamental action of antimicrobial stewardship programs (ASPs). Reference Dellit, Owens and McGowan1 ASPs have played essential roles in coronavirus disease 2019 (COVID-19) response efforts since the onset of the pandemic. For instance, ASPs have implemented the preauthorization of remdesivir throughout its path from an experimental antiviral obtained via compassionate use or expanded access, to Food and Drug Administration (FDA) Emergency Use Authorization (EUA), to ultimate FDA approval. Reference Stevens, Patel and Nori2,Reference Mazdeyasna, Nori and Patel3 On November 9, 2020, the FDA released an EUA for bamalanivimab, a recombinant human monoclonal antibody to the spike protein of SARS-CoV-2. 4 Unlike previous EUAs for remdesivir and convalescent plasma, the bamalanivimab EUA is intended for use in outpatients with mild-to-moderate COVID-19 to avoid hospitalizations due to disease progression. 4 What role, if any, should ASPs play in bamlanivimab preauthorization? Notably, preauthorization is not commonly deployed by ASPs in outpatient stewardship efforts Reference Drekonja, Filice and Greer5.
ASPs are well suited to lead preauthorization efforts given their existing experience and infrastructure. Reference Stevens, Patel and Nori2 Preauthorization is crucial for a scarce experimental product with limited available supporting evidence in high public demand. Preauthorization is also a critical component of equitable distribution to patients who may maximally benefit. For this process to function optimally, a 24-7 preauthorization structure is ideal but likely is not feasible at most centers. Importantly, time invested in outpatient bamalanivimab preauthorization will siphon effort away from other essential ASP functions. Reference Mazdeyasna, Nori and Patel3
Another critical role ASPs can play is to lead discussions about the ideal deployment of bamalanivimab locally. Given the limited available literature, including several inpatient studies of monoclonal antibodies that were halted due to unfavorable data, Reference Chen and Heller6-8 it may be prudent to await further data and/or guidance from professional organizations (eg, the Infectious Diseases Society of America). See Figure 1 for additional considerations.
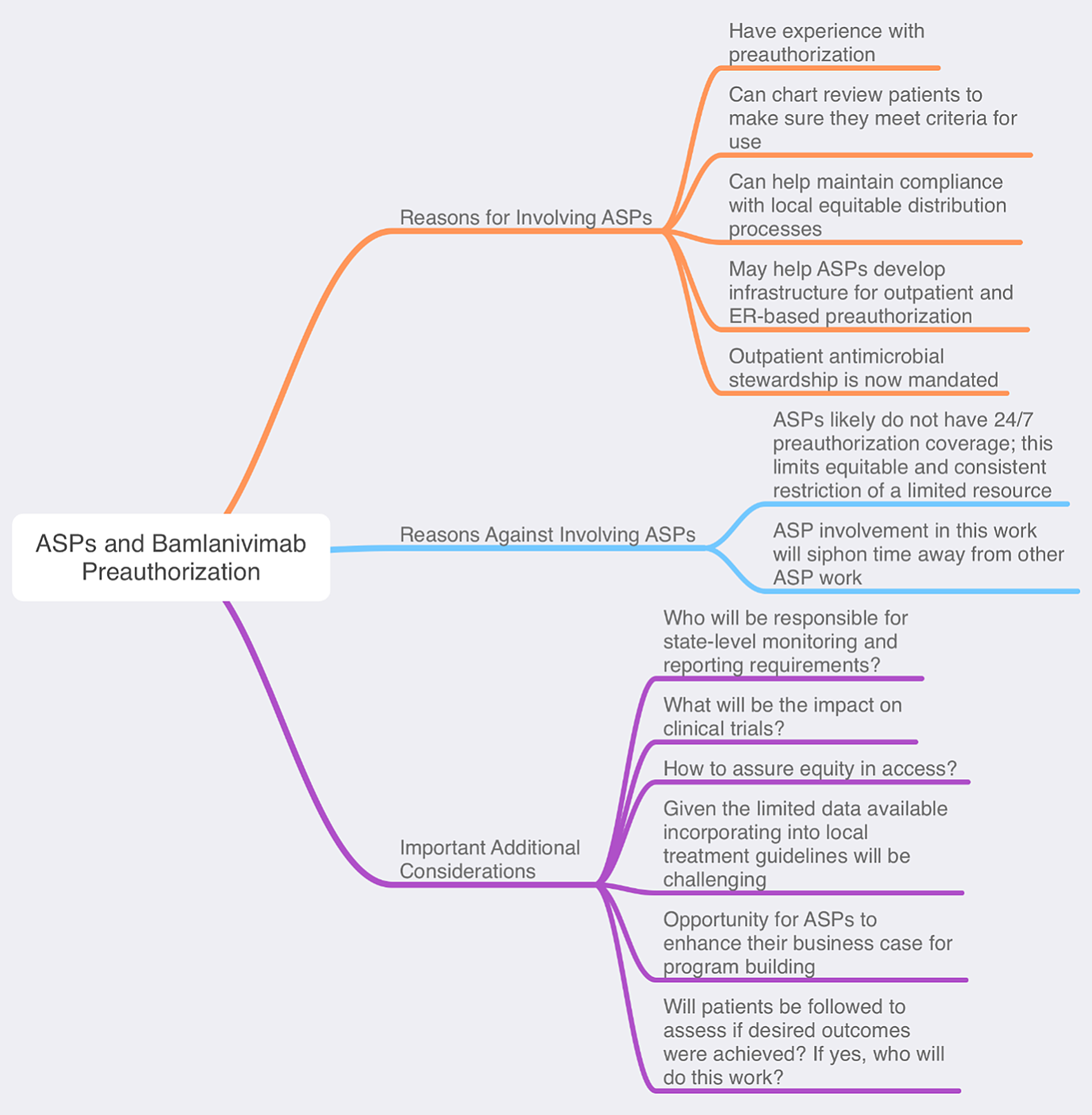
Figure 1. ASPs and Bamlanivimab preauthorization
The bamlanivimab EUA may present ASPs with a golden opportunity to enhance their outpatient stewardship impact. As of January 1, 2020, the Joint Commission has mandated that health systems deploy active antimicrobial stewardship interventions in ambulatory settings. 9 Bamlanivimab preauthorization can serve as a vehicle for programs to meet the new requirements if administered in outpatient clinics. Beyond this, the infrastructure developed may be harnessed later for other high-value outpatient stewardship targets (eg, long-acting anti-MRSA agents and oral carbapenems). ASPs can develop or strengthen collaborations with emergency departments or urgent-care centers to create a SARS-CoV-2 test, treat, and release pathway. In turn, this could help relieve inpatient overcrowding, which has been a national issue in the pandemic. Additionally, telehealth platforms can be utilized for close patient follow-up, potentially increasing revenue and saving costs for hospitals and ASPs.
Perhaps the most critical role of antimicrobial stewards at this juncture is to instill experience and expertise into the process of evaluating the role of bamlanivimab in patient care. If deployed, ASPs can help identify who, when, and where the drug should be given to, and they can help assure compliance with identified best practices. This EUA is further evidence that health systems should invest in their ASPs, for which roles are expanding with each new agent in the COVID-19 armamentarium.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.




