INTRODUCTION
Hepatitis C virus (HCV) is a major public health problem [1] with an estimated 180 million people or 3% of the world's population currently infected [Reference Pybus2, Reference Perz3], giving rise to an estimated annual toll of 366 000 deaths [Reference Ali4], mainly due to cirrhosis and primary liver cancer. Previous studies, mostly conducted in large urban centres, have shown that Pakistan is a high prevalence country [Reference Ahmed5–Reference Qureshi13] with 3–8% of the general population, and 2% of pregnant women being infected [Reference Jafri6, Reference Khan8, Reference Afridi11]. Major risk factors identified in Pakistan include the re-use of unsterile injection equipment (Pakistan has one of the highest global rates of use of therapeutic injections) [Reference Janjua, Akhtar and Hutin14] and other iatrogenic exposures such as unscreened blood transfusions [Reference Ali4, Reference Ahmed5, Reference Khan8, Reference Akhtar and Moatter12].
There are significant social and economic differences across Pakistan's four provinces of Balochistan, Sind, Punjab and Khaiber Pakhtoonkhuah. Kech District, located in the South West, is the second largest by population in Balochistan. There are a number of additional problems in Kech compared to the major urban centres in Pakistan which may increase the risk of bloodborne virus infections. These include (i) it is on a main supply route of opium from Afghanistan to the rest of the World, making injectable drugs potentially readily available [15], (ii) there is a highly mobile workforce with large numbers of young adult males working particularly in Gulf countries, (iii) a lower than average literacy rate and socioeconomic conditions compared to the rest of Pakistan, (iv) poor access to healthcare services with inadequate screening of transfused blood, and (v) local embroidery of cloths which can lead to occupational exposure when these needles are shared.
Given the lower socioeconomic circumstances healthcare utilization is likely to be different; in particular lower use and poorer quality of secondary care, either higher (unable to afford new syringes and needles) or lower (unable to afford medicine) use of unclean injection equipment [Reference Ali4] and less screened blood used for transfusion. We therefore undertook a community-based cross-sectional study of the prevalence of, and risk factors for, hepatitis C infection in Kech in order to determine whether the epidemiology and risk factors for HCV in this poor, rural area are similar to those reported in previous studies in other areas of Pakistan.
METHODS
Setting and population
A cross-sectional study design was conducted during 2007 to 2009 in Kech, a remote district of Balochistan province covering 22 539 km2 with a population of around 750 000, 88% of whom live in rural areas. Kech District has four sub-divisions, Turbat, Tump, Dasht and Buleda. These are divided into 38 Union Councils and 454 blocks. Ninety-nine percent of the population is Muslim and the main industry is agriculture. Kech is a below average area for socioeconomic development.
Sample unit recruitment was based on the voting areas (454 blocks). The numbers of voters with their names (electoral roll) were obtained from the Election Commission Office of Turbat. All blocks have similar population sizes. The random number function in Microsoft Excel 2003 was used to randomly select 30 blocks; 18 from Turbat, three from Tump, five from Dasht, and four from Buleda areas. Households were randomly selected from within these blocks using the same method.
Data collection
A risk factor data questionnaire was developed using two previously used questionnaires [Reference Neal16, Reference Murphy17] with the addition of local knowledge and other published data with special reference to Pakistan. The questionnaire included questions on age, gender, occupation, past medical health, use of alcohol, tattooing, use of razors, street barbers, piercing, use of illegal drugs, prison and sexual history. Healthcare workers included doctors, dentists, nurses, healthcare technicians and emergency department staff.
The data collection was undertaken between 2 September 2007 and 17 June 2009. One author (F.A.) and one trained female health technician were involved in supervising the collection of the data and blood samples.
Household setting
Sampling commenced in the middle of the block and moved around according to the electoral roll, with a three-house gap between two selected households, i.e. selected houses being the first, fifth, ninth, etc. The head of the family was approached and informed consent obtained. The questionnaire was administered and a venous blood sample collected. If there was no reply from a house, or no consent, the next house was visited. Between 60 and 70 people were recruited from each block. Only five households declined to participate.
From each house we included the householder and their spouse, one male child of the householder, one female child of the householder, and the householder's parents (if present). Only people aged ⩾18 years and ⩽96 years were included and 2000 people were recruited.
Blood sampling and testing
During the survey 4 ml blood was collected into a plain tube. Serum was separated by centrifugation at 4°C and stored in two labelled sterile tubes at −40°C within 6 h of collection. Serological testing was performed according to manufacturer's instructions (anti-HCV antibodies ETI-AB-HCV-4, No. 146; Dia Sorin Italy). Blood test results were given to study participants in sealed envelopes at the end of the study. Borderline results were re-tested using the same methods.
Data entry
All questionnaire data was entered into a Microsoft Access 2003 database and then rechecked by two of the authors (F.A., M.A.). Results of hepatitis C testing were obtained from the laboratory and linked to the questionnaire data by study number.
Statistical analyses
Univariate analysis was performed using logistic regression with hepatitis C antibodies as the outcome variable. Potential risk factors explored included age, gender, healthcare occupation, needle-stick injuries, surgical procedures, dental treatment, therapeutic injections and vaccinations, tattoos, ear or nose piercings, circumcision, blood transfusions, imprisonment, acupuncture, sexual history, sharing of razors, history of injecting drug use and sharing of needles. All exposure variables were coded as categorical variables. For ordered categorical variables, P for trend was calculated by including these as continuous variables in the regression model.
Results are presented as unadjusted odds ratios (OR) and 95% confidence intervals (95% CI). A P value of ⩽0·05 was considered statistically significant. Subsequently, a multivariable regression analysis was conducted by including all statistically significant variables (at the 5% level as identified in the univariate analysis) in the multivariable model. A separate dummy category was created for missing variables for inclusion in the analyses. In most cases this category was automatically excluded in the analysis because of nil observations in one of the categories. All statistical analyses were conducted using Stata version 11 (StataCorp, USA).
Ethical approval
The study protocol conformed to the 1975 Declaration of Helsinki and was approved by the appropriate ethics groups for Ata Shad College, Turbat and the Kech Health Department.
RESULTS
Evidence of hepatitis C infection was found in 110/2000 persons (5·5%, 95% CI 4·5–6·5). Adjusting for the lower number of males recruited the prevalence becomes 6·0%. Univariate risk factors are shown in Table 1. Male gender, older age (⩾75 years), being a healthcare worker, history of jaundice, alcohol use, a tattoo done by friend or self, sharing a razor, injecting drug use and sharing needles were all associated with HCV infection with ear and nose piercing appearing protective. The independently positive associations demonstrated by multivariable analyses were male gender, age ⩾75 years, being a healthcare worker, and injecting drug use and are shown in Table 2.
Table 1. Univariate analysis of risk factors for HCV infection (n=2000)
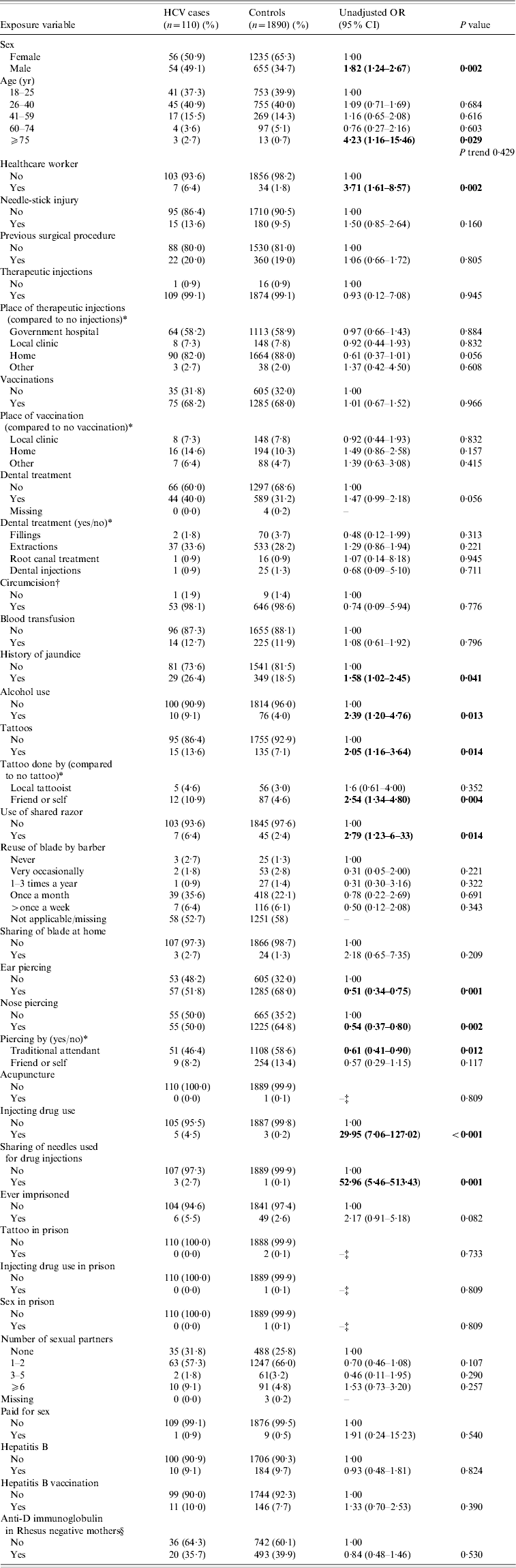
Summary of results. Statistically significant risk factors for HCV: healthcare workers, males, age ⩾75 years, history of jaundice, alcohol use, tattoos (especially those done by friends or self), use of shared razors, injecting drug use, sharing needles when injecting drugs. A statistically significant decrease in HCV was seen in subjects with nose and ear piercings. This could simply be a reflection of the lower risk in females as nose/ear piercings are more prevalent in this group.
OR, Odds ratio; CI, confidence interval.
Statistically significant results in bold (significant at P⩽0·05).
* Categories are not mutually exclusive.
† Subgroup analysis of only males in the sample.
‡ Could not be calculated.
§ Subgroup analysis of only females in the sample.
Table 2. Multivariable analysis of risk factors for HCV infection (n=2000)
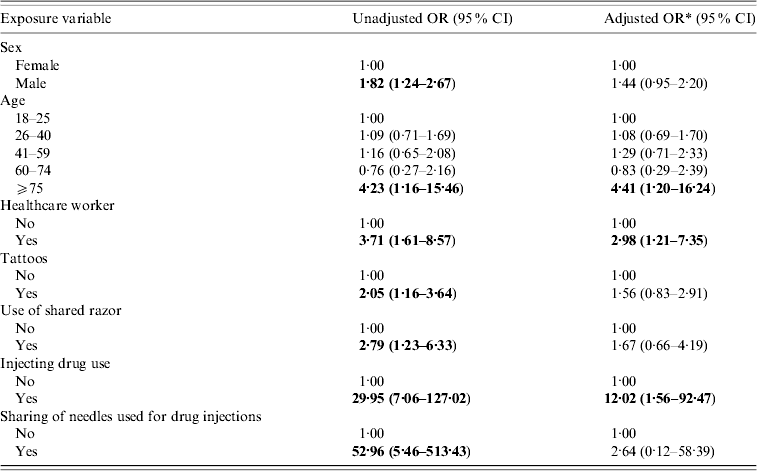
Summary of multivariable analysis results for HCV. Statistically significant risk factors for HCV: healthcare workers, people in the ⩾75 years age group and injecting drug use.
OR, Odds ratio; CI, confidence interval.
Statistically significant results in bold (significant at P⩽0·05).
* Adjusted for all other variables in the model.
For females, independent risk factors were dental treatment and having a tattoo and for males independent risk factors were being a healthcare worker and injecting drug use.
DISCUSSION
This is the first study of HCV epidemiology and risk factors conducted in Kech District, Balochistan, Pakistan. The random selection from the complete electoral roll means that the population is representative except that men were under-represented as they were out at work. The prevalence for males is 7·62% (95% CI 5·5–9·6) and reweighting gives an adult population prevalence of 6%. The exclusion of children and the highest risk seen in older people raise the possibility that this prevalence may decline over time. Although we were only able to repeat borderline results due to resource constraints; given that the sensitivity and specificity of the assay used was in the high 90s and even as high as 99% we can be confident regarding the accuracy of the overall results. Consequently, there would be very little misclassification bias in our results which would have very little effect, if any, on our overall findings.
The high rates seen in older people was based on small numbers, but given that older people have had more time to acquire HCV this is likely to be a valid finding. It is also possible that given the higher mortality seen in HCV-infected people in Western countries [Reference Neal18] where better healthcare of liver disease is provided, infected people may have died earlier, especially if they acquired the infection in childhood, hence under-estimating the problem. In addition prevalence of key risk factors may have altered over time and changes in technology such as disposable syringes and better awareness of risks could have had led to major changes in infection risk.
Risk-factor data was based on a questionnaire we had successfully used in two previous studies [Reference Neal16, Reference Murphy17], so the data collection methods were valid. We used local knowledge and other published data with special reference to Pakistan to make the questionnaire culturally acceptable as well as inclusive of local risk factors. The women were interviewed by a female interviewer reducing anticipated difficulties in answering the questions on sexual behaviour. Although a number of women reported more than one sexual partner, these partners were all husbands. No such difficulty exists for reporting male sexual behaviour in Pakistan [Reference Rajabali19]. Blood testing was performed by an accredited laboratory using a routine ELISA method, which is unlikely to lead to any serious risk of misclassification that would weaken our results.
Only eight study participants admitted to recreational injection of drugs, but even so, injecting drug use was a highly significant risk factor for infection. The fact we were able to identify this risk factor given the legal framework in Pakistan adds validity to our other results. Due to Kech being on a main worldwide supply route of drugs to the coast (900 km long), means the numbers of injecting drug users in the study area are increasing. Tattooing by a friend and self has also been previously shown to be an important risk factor for HCV infection [Reference Neal16, Reference Murphy17]. In univariate analysis, being a healthcare worker was a significant risk factor, which has also been shown in other studies. This again is not surprising, given the prevalence of HCV in the community and the poor standards of infection control, such as lack of gloves and disposable equipment.
We were unable to identify major risk factors in the majority of people who tested positive. This may be due to the high prevalence of certain practices such as the use of therapeutic injections and dental treatment [Reference Qureshi13], the majority of which will have been performed using methods which can readily transmit bloodborne viruses. In this regard, it is of interest to note a clear trend towards the use of therapeutic injections at home (P=0·056), and dental treatment (P=0·056) as risk factors.
Throughout Balochistan there are two particular traditions that are potential factors, especially in females. First is ear and nose piercing. We were unable to demonstrate that this carried any increased risk of infection, but this may reflect the almost universal exposure to these practices. Second is the major local industry of embroidery where there is potential for pricking fingers with needles that are usually shared and which may therefore contribute to the spread of HCV and other bloodborne viruses.
CONCLUSION
This is the first large rural, community-based study in Kech District, Pakistan. We demonstrated a high prevalence of HCV infection and confirmed the public health importance of HCV. Although our study showed the importance of certain infection routes, we were unable to identify the route of infection for most people infected. Almost all individuals, whether infected or not, had potential risk factors for bloodborne viruses. At the population level most HCV infections remain unexplained although there are many possible routes of infection. As a result, reducing infection will require a range of efforts.
ACKNOWLEDGEMENTS
We gratefully acknowledge the financial support provided for this study by the Higher Education Commission of Pakistan and the British Council (Karachi, Pakistan). We acknowledge the team (institution's academics) directly involved in the data collection, the cooperation of students and other friends for their help in the study and the participants in Kech District.
DECLARATION OF INTEREST
None.






