INTRODUCTION
Botulism is a severe neuroparalytic disease that affects humans, all warm-blooded animals and some fishes. The disease is caused by exposure to botulinum neurotoxins (BoNTs) produced by anaerobic, spore-forming, ubiquitous bacteria of the genus Clostridium, referred to as BoNT-producing clostridia [Reference Smith, Hill and Raphael1]. BoNTs interfere with acetylcholine release at the neuromuscular junction, which causes a progressive, symmetrical, flaccid muscle paralysis that leads to recumbency and death. Whereas human botulism is quite rare and well managed nowadays; botulism in animals represents a serious welfare and economic concern because of limited options for treatment and the subsequent high mortality rate [Reference Critchley2, Reference Skarin3]. Knowledge about potential mechanisms of transmission is therefore crucial to effectively prevent outbreaks.
BoNT-producing clostridia comprise six phylogenetically distinct clostridial groups: Clostridium botulinum groups I, II, III; Clostridium argentinense; some strains of Clostridium butyricum and Clostridium baratii [Reference Smith, Hill and Raphael1, Reference Rossetto, Pirazzini and Montecucco4]. These bacteria can survive for many years in the form of highly resistant spores in soil and aquatic sediments, as well as in the gastrointestinal (GI) tracts of livestock animals, wild birds and fishes [Reference Meyer5–Reference Notermans, Dufrenne and Oosterom7]. When the conditions are suitable, the spores develop into vegetative forms and may produce one of the seven serotypically distinct BoNTs, denoted A to G. Suitable growth conditions can be defined in general as anaerobic conditions with a protein source, sufficient temperature and moisture, low salinity and low acidity. For animals, these conditions can be found in decaying carcasses, insufficiently acidified silage or decomposing vegetation in ponds [Reference Deprez, Mainil, Duschesnes, Granum and Menozzi6]. In addition, in types C and D, some strains are a mosaic D/C or C/D form, which possesses gene coding for two-thirds of the type D (or C) toxin and gene coding for one-third of the type C (or D) [Reference Moriishi8].
Susceptibility to BoNTs seems to be highly variable among the different animal species. Human botulism cases are mainly due to serotypes A, B, E and F – produced by group I and II organisms, C. butyricum and C. baratii – whereas reported animal cases are mostly associated with serotypes C and D produced by group III organisms.
The route of contamination in cattle is assumed to be primarily the ingestion of preformed toxins in food, water or carrion [Reference Deprez, Mainil, Duschesnes, Granum and Menozzi6, Reference Lindström9]. The first outbreaks reported were mainly attributed to bones licked by cattle grazing in phosphorous-deficient areas [Reference Theiler10, Reference Brizuela11], ingestion of water or silage contaminated by small mammal or bird carcasses [Reference Myllykoski12, Reference Doutre13], use of poultry litter as a dietary supplement [Reference Jean14, Reference McLoughlin, Mcllroy and Neill15], ingestion of insufficiently acidified silage contaminated by telluric C. botulinum spores [Reference Kelch16, Reference Divers17], or feeding silage made from contaminated brewers’ grains [Reference Notermans18]. In the past few decades, an increasing number of cattle botulism type D outbreaks linked to direct contact with or close proximity to poultry litter has been reported in Western Europe [Reference Payne19–21]. Most of these outbreaks have been attributed to the ingestion of BoNTs from poultry or small mammal carcasses that were present in poultry litter spread as fertilizer or stacked onto pastures, but the causal association has not always been proved [21–Reference Hogg, White and Smith23].
In spring 2015, a large outbreak of type D/C bovine botulism occurred on a farm with dairy and poultry operations in Western France. The objective of this study was to investigate the mode of exposure of cattle, with particular emphasis on the potential role of poultry litter and carcasses.
METHODS
Outbreak description
An outbreak of botulism occurred in spring 2015 on a farm with dairy and poultry operations in Western France. Dairy cattle, broilers and laying hens were reared at four different sites approximately 600 m apart. Milking cows were reared at site 1, grazed in summer and housed in a cubicle house during the winter period. The herd was divided into two feeding groups according to the level of production (LP: low-producing cows, HP: high-producing cows), and fed a mixed ration based on maize and grass silage. Each group was conducted separately with different feeding regimen and different access to pastures, in terms of date of access and patch of pasture grazed.
From 20 March (day 1 of the outbreak) to 11 May (day 57), 80 of 110 Holstein cows died after having developed signs of muscle weakness that began in the hind limbs and gradually led to sternal and then lateral recumbency. At least three cows also had dysphagia, with difficulty swallowing and excessive salivation. No flaccid tongue was observed. Clinical signs progressed rapidly and death occurred on average within 48 h (ranging from 3 h to 8 days), despite supportive treatments.
Vaccination against C. botulinum was initiated on day 51, with a second injection on day 85, using a bivalent C and D botulinum toxoid (Ultravac® Botulinum, Zoetis France) available in France under emergency drug release. All cows introduced after that date were vaccinated with a primary series on arrival. Two additional cows died on days 75 and 88 after showing the same clinical signs.
No calves, heifers or bulls exhibited any clinical signs, nor did the two flocks of poultry broilers or the flock of laying hens.
Necropsy and sampling of animal biological material
Two carcasses were sent on day 7 to the regional veterinary laboratory and two others on day 40 to the University Veterinary Hospital (Oniris, Nantes, France) for necropsy and laboratory investigations.
Routine necropsic examination was performed and samples of intestinal content were sent to the Pasteur Institute (Paris, France) for C. botulinum examination. The serum of 10 other affected cows was sent to the Pasteur Institute for C. botulinum examination on day 40. Samples of intestinal content of one cow were sent to the Departmental Veterinary Diagnostic Laboratory (Inovalys, Nantes, France), for C. perfringens examination on day 40.
Epidemiological investigation
The local veterinary practitioner requested assistance from the Veterinary University to identify the source of contamination. The farm was visited by the first author and the local veterinarian 94 days after the onset of the outbreak. We examined the distribution of events over time and space, silage collection, feed and water distribution, pasture fertilization, as well as the management of poultry carcasses and poultry litter.
Food specimen collection and processing
The silages used to feed dairy cattle were observed during the farm visit (day 94). Silage suspected to be involved as a medium of C. botulinum or its toxin was sampled on day 106 by the veterinary practitioner (50 g of silage, sampled in three anaerobic areas of the silage), and sent in a closed plastic tube at ambient temperature to the Pasteur Institute (Paris, France) for C. botulinum examination.
Laboratory analysis
C. perfringens bacteria and toxins (α, β, ε) were searched by ELISA (Bio X DiagnosticsND, Jemelle, Belgium).
Detection of BoNT in animal and food samples was based on a mouse lethality assay. The tests were performed in accordance with the European Directive 2010/63/EU on the protection of animals used for scientific purposes (laboratory animal use agreement n° 2013-0116). Botulinum toxin from 10 g of food samples was extracted by incubating the sample 30 min at room temperature in 20 ml of 50 mM phosphate buffer (pH 6·5) containing 1% gelatin. The extract was then centrifuged end filtered and 0·5 ml was injected intraperitoneally into Swiss mice weighing 20–22 g (Charles River Laboratories, l'Arbresle, France). The mice were observed for up to 4 days for the presence of typical clinical signs (pinching of the waist, laboured breathing and paresis) and euthanized immediately after observation of such signs. BoNTs were confirmed, and types were identified using a seroneutralization test on mice with specific botulinum antitoxins for types C and D from the National Reference Center for Anaerobic Bacteria and Botulism [Reference Lindström and Korkeala24, Reference Fach25].
Detection of C. botulinum in food and bovine intestinal contents and blood samples was based on culturing samples in freshly prepared fortified cooked meat medium at 37 °C under anaerobic conditions [Reference Takeda26]. After a 48 h period of incubation, 1 ml of enrichment culture was collected and DNA extracted from the pellet cells using a QIAamp DNA Stool Kit (Qiagen) according to the manufacturer's instructions. Detection of BoNT genes (bont), namely bontC, bontD, bontC/Dand bontD/C, was performed by SYBR green real-time PCR using the following primers and according to the protocol previously described [Vanhomwegen et al., PLoS ONE. 2013; 8(6): e67510]
bonT/C: P1652 GGCACAAGAAGGATTTGGTG P1653 TTGGATCCATGCAAAATTCA
bont/D: P1654 TTGGGCGAATGAAGTAGTTG P1655 TCCCCTTAATGCTGAATTTCC
bont/CD: P1795 TGAAAATGCATACACGCCAGT P1796 TACCCATCCTGGATCCCTAGA
bont/DC: P1797 CCACTAGGTGCATTGGATCA P1798 TCCAAGCCTATTTGTTGTTGC.
The presence and the type of viable and toxic C. botulinum strains in the samples were confirmed by the detection of botulinum toxin in the culture supernatant performed by the mouse lethality and seroprotection assay after 4 days of enrichment cultures.
RESULTS
Necropsy findings
No significant gross lesions were found, except for petechiae and suffusions on mucosal and serous surfaces for two cows (day 40), as well as congestion of the abomasal mucosa and segments of the small and large intestines for one cow (day 40). None of the cows were observed with intestinal haemorrhage or decayed carcass material in their stomach contents.
Distribution of events in space and time
Figure 1 represents the patches of pastures grazed by the cows during the outbreak. Figure 2 represents the evolution in time of deaths, the grass silage fed (A or B) and the patch of pasture grazed.
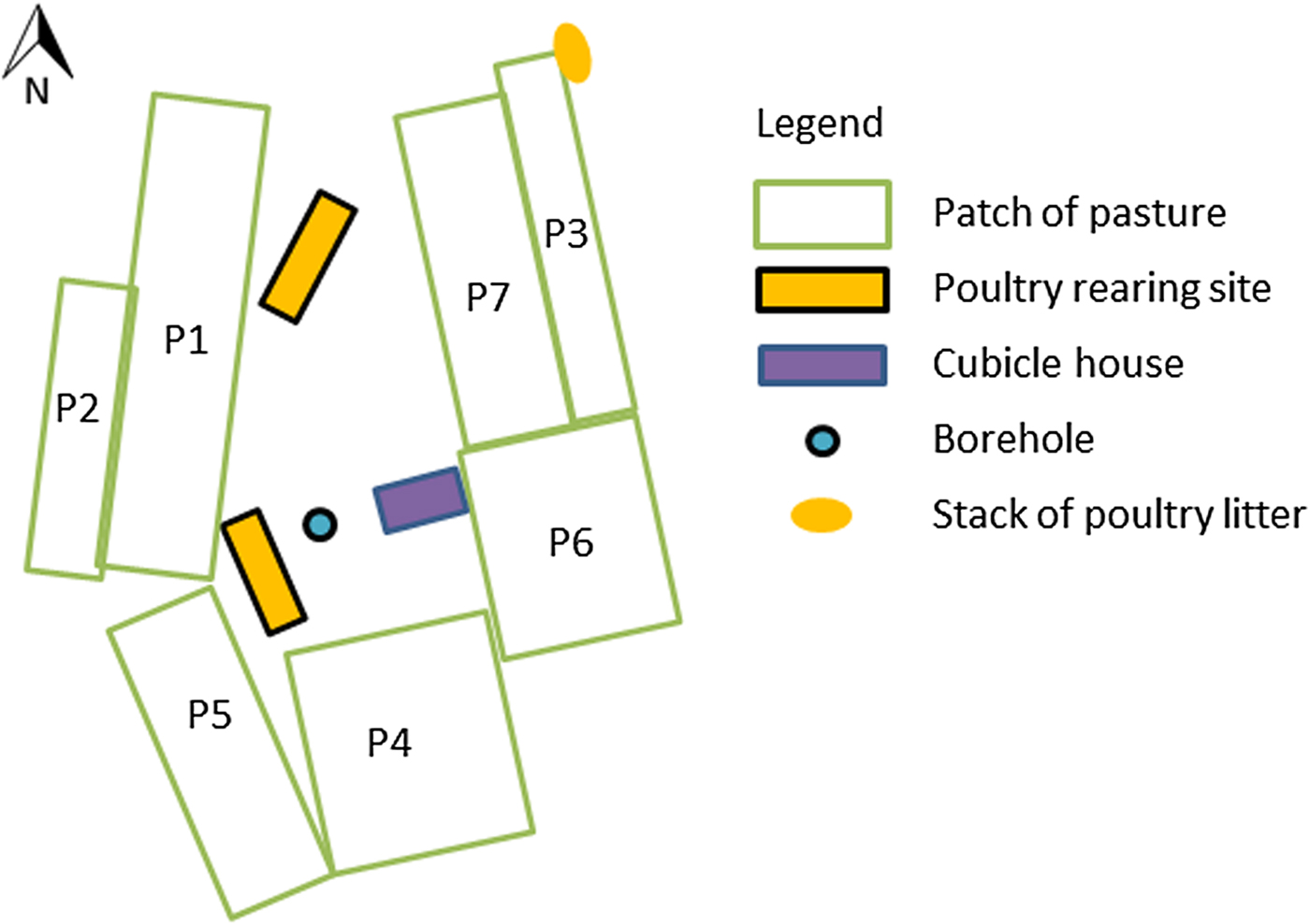
Fig. 1. Layout of the dairy farm site (site 1) that experienced a massive outbreak of type D/C botulism in Western France in spring 2015.
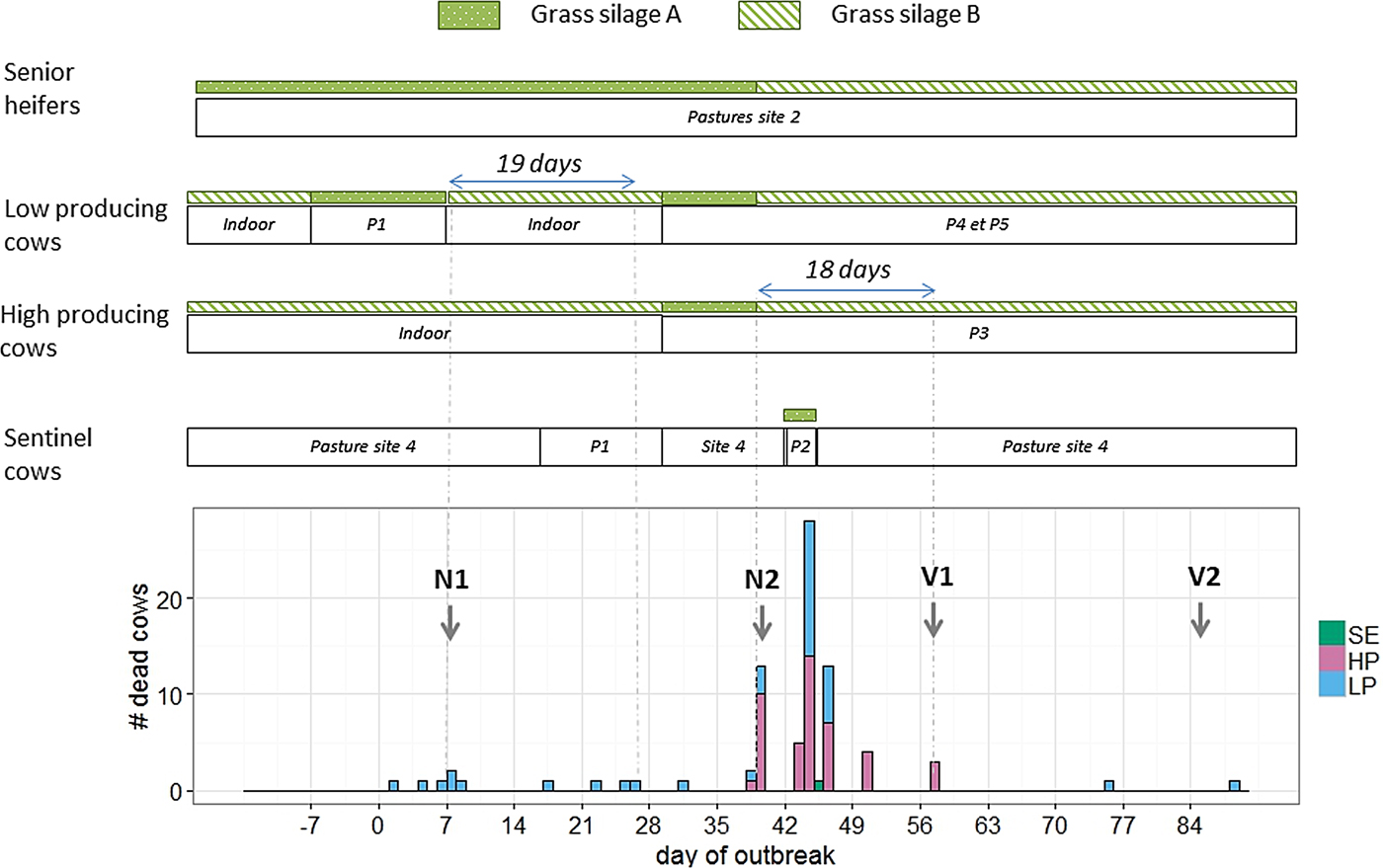
Fig. 2. Distribution of events in time and space during an outbreak of type D/C botulism in a Holstein dairy herd in Western France in spring 2015 (P: patch of pasture at site 1; N: necropsy; V: vaccination against Clostridium botulinum; SE: sentinel cows; HP: high-producing cows; LP: low-producing cows).
Before the onset of the outbreak, all milking cows and yearling heifers were reared indoors at sites 1 and 3, respectively, and fed grass silage B, whereas senior heifers grazed at site 2 and were fed grass silage A.
Deaths occurred in two waves. The first lasted from day 1 to day 26 and affected only cows in the LP group (10 deaths). Four cows were placed by the farmer as sentinel animals between days 16 and 29 on a patch of pasture previously grazed by the affected cows, without exhibiting any clinical signs. The second wave lasted from day 31 to day 57 and affected both groups (LP and HP) of cows (70 deaths). The four sentinel cows were moved to a patch of pasture not previously used and fed grass silage A from day 42 to day 45. One of them died on day 45.
Except for the two late cases, all deaths occurred within 19 days after the ingestion of grass silage A (Fig. 2). The cow that died on day 75 had not been vaccinated as she should have been culled earlier. The cow that died on day 88 calved on day 49, had received two injections of the vaccine and had never been fed grass silage A.
Senior heifers were fed with grass silage A until day 39 without exhibiting any clinical signs.
The bulls and calves were not fed any grass silage.
Silage collection, feed and drinking-water distribution
Grass silage A was prepared from a pasture located 4 km away. Forage was sown on 10 July 2014, harvested on 15 September 2015 and stored in a 7 × 6 m bunker silo with concrete floor at site 2. The weather prevented the farmer from waiting for normal pre-wilting. At the end of storage, the silage stack was 1·5 m high. The first ration containing grass silage A was distributed to the senior heifers at the beginning of winter. When the clinical signs began, 7 days after its distribution to LP group, the silage stack was around 4 m in length and 1 m high, or about 28 m3. When it was analysed for botulism, only about 6 m3 remained.
Grass silage B was prepared from a pasture located close to site 2. Forage was sown in August 2013, harvested in May 2014, and stored after pre-wilting in a 9 × 16 m bunker silo with concrete floor at site 3. No additive was used.
Both silages were comprised of ryegrass, clover and hairy vetch. They were covered with a standard black plastic sheet (150 µm thick) and tyres.
Feed was mixed manually for heifers and bulls, but with a mixer for milking cows. A hay bale was distributed weekly to milking cows. The farmer reported that he had observed some refusal of grass silage A by the senior heifers.
All cattle, heifers, bulls and calves drunk water from a water borehole located on site 1 (Fig. 1).
Pasture fertilization
All patches of pastures at site 1 were fertilized with dairy cattle manure on 15 March 2015, except patches 1 and 2, which had not been fertilized in the previous years. The manure came from the farm. Silage pastures were fertilized with a mineral fertilizer.
Management of poultry carcasses and poultry litter
Poultry flocks were checked daily for the occurrence of mortality and any carcasses were immediately removed, stored in a freezer and disposed of in a rendering container before rendering plant pick-up.
Poultry litter was stored and stacked alongside pastures. One stack was stored next to grazing pastures at site 1 and separated by an electric fence to prevent access by grazing cattle (Fig. 1). This stack was collected by a contractor every 2 weeks. The prevailing wind direction is west to east, but an east to west wind blew on several occasions before and during the outbreak. Dispersion of litter particles by wind overgrazing pastures cannot be excluded, but contamination of the patch of pasture grazed at the onset of the outbreak (patch 1) is unlikely, as this patch is protected from the easterly wind by the poultry housing (Fig. 1). The stack of poultry litter was stored slightly below the pastures, which should have prevented flowing water from carrying litter particles towards grazing pastures.
Two stacks of poultry and dairy cattle litter were stored in the middle of silage pastures to be used as fertilizer on maize crops (pasture located 4 km away from which grass silage A was prepared). These stacks had been stored for the duration of grass growth and were present during harvesting of grass silage A.
Observation of grass silage
No carcasses were observed in any silage by the farmer. During the farm visit on day 94, the two grass silages were inspected. Grass silage A was a very dark olive green, moist, with a very offensive odour (a putrid ammonia smell). Grass silage B was straw yellow, with a slightly sweet acidic smell (Fig. 3).

Fig. 3. Observation of two batches of grass silage fed during an outbreak of type D/C botulism in a Holstein dairy herd in Western France in spring 2015. The silage A was harvested in September 2015 and the silage B in May 2014.
As grass silage A was the most likely medium of C. botulinum or its toxin, it was sampled on day 106 and sent to the Pasteur Institute (Paris, France) for C. botulinum examination.
Laboratory results
C. botulinum type D/C bacteria were detected in a sample of intestinal content from one of the four cows (day 40) by PCR and mouse bioassay after anaerobic enrichment. No BoNT was detected either in the samples of intestinal contents from the four cows or in the serum of 10 other affected cows. C. perfringens was detected in the intestinal content of the cow examined (day 40), but not its toxins (α, β, ε).
C. botulinum type D/C bacteria were detected in grass silage A by PCR and mouse bioassay after anaerobic enrichment. No BoNT was detected in the grass silage.
DISCUSSION
In the past few decades, an increasing number of type D botulism outbreaks have been reported in cattle in Western Europe and were linked to contact with or close proximity to poultry litter. The presence of carcasses of birds that died during production was regarded as the likely source of BoNTs, the cattle being exposed through direct ingestion of litter, ingestion of particles containing BoNTs spread onto pastures by scavengers, or ingestion of grass silage fertilized with poultry litter [Reference Payne19, 21]. This study is the first to suggest that large amounts of grass silage can be contaminated with type D/C C. botulinum spores in the absence of pasture fertilization with poultry litter, and that poultry litter may be at risk even with proper management of poultry carcasses.
The definite diagnosis of botulism should rely on the detection of BoNTs by mouse bioassay in serum, ruminal fluid, intestinal contents of tissue of affected animals [Reference Lindström and Korkeala24]. However, definite diagnosis is usually difficult in cattle as BoNTs are rapidly degraded by ruminal microbes [Reference Allison, Maloy and Matson27] and in post-mortem tissue [Reference Brooks28], and BoNTs concentration in the serum of clinically affected cattle may be below the threshold of detection of the bioassay [Reference Kelch16], giving false-negative results. Diagnosis of bovine botulism thus mostly relies on the observation of typical signs and ruling out of other potential causes of the problem [Reference Le Maréchal, Woudstra, Fach, Uzal, Songer, Prescott and Popoff29]. A circumstantial diagnosis can be made by finding the organism and BoNTs in foodstuffs that recently have been consumed by the animals exhibiting clinical signs of botulism or by test feeding of suspect toxic feed to susceptible animals [Reference Lindström and Korkeala24, Reference Le Maréchal, Woudstra, Fach, Uzal, Songer, Prescott and Popoff29].
In this case, the typical clinical signs of botulism were observed in cows in the 18 days following the ingestion of grass silage A; this silage was dark green, moist and had a really unpleasant odour, which is characteristic of clostridial contamination [Reference Jones30, Reference Seglar and Shaver31]; the viable type D/C C. botulinum was detected by PCR and mouse bioassay in the grass silage A and the intestinal content of one affected cow. Moreover, one of the four sentinel cows showed signs of botulism and died after 3 days of consumption of this grass silage. All of these findings confirm the diagnosis of botulism and strongly suggest the implication of this silage as the medium that allowed growth and toxin production from C. botulinum spores [Reference Lindström and Korkeala24, Reference Jones30].
While the accidental contamination of the grass silage by soil, animals trapped during harvesting or burrowing animals that died in the stored silage should have spoiled only a pocket of the silage, the detection of type D/C C. botulinum in grass silage at the end of the outbreak, after at least 15 days of distribution and more than 20 m3 distributed, suggests massive contamination of the silage before harvesting. Since no poultry manure had been used as a fertilizer on silage pastures, the most likely mode of silage contamination is the dispersal of particles containing C. botulinum spores by wind or runoff water from a stack of poultry litter that was stored alongside the pasture before silage harvesting. Unfortunately, this stack was not present at the time of the epidemiological investigation and could thus not be tested for the presence of C. botulinum spores or toxins.
Two sources have been previously proposed to explain the contamination of poultry litter by C. botulinum spores and toxins: the presence of putrefied poultry carcasses mixed in with the litter, or the presence of rodents that were attracted by the litter and died in it [Reference Payne19, 21, Reference Smart22]. Particles of litter containing the spores or the BoNTs could then be dispersed in the silage pasture by wind, runoff water or scavengers [21, Reference Carlier and Popoff32]. In the case in question, the presence of putrefied poultry carcasses mixed in with the litter is quite unlikely as the litter came from the farm, which has good management of poultry carcasses, in other words daily inspections and the removal and safe storage of dead birds [21]. The presence of rodent carrion in the litter cannot be excluded, but was not observed by the farmer. Another source can be hypothesized: spores of type D/C C. botulinum could have been present in the litter due to asymptomatic carriage in the poultry gut, as has been observed with type B and C C. botulinum in pigs [Reference Myllykoski33, Reference Yamakawa34]. Indeed, poultry are less susceptible than cattle to type D botulism [Reference Le Maréchal, Woudstra, Fach, Uzal, Songer, Prescott and Popoff29, Reference Gross and Smith35] and the mosaic D/C form, which possesses gene coding for two-thirds of the type D toxin and gene coding for one-third of the type C toxin [Reference Moriishi8], could be particularly adapted to their carriage by birds [Reference Rossetto, Pirazzini and Montecucco4, Reference Moriishi8, Reference Woudstra36]. Under this scenario, the occurrence of botulism in cattle would require the growth of spores in adequate substrate, which, in the present outbreak, would be the insufficiently fermented grass silage. Further research on the asymptomatic carriage of type D/C C. botulinum by poultry would help to better assess the risk of exposure of cattle to C. botulinum spores associated with poultry litter. In any event, recommendations for ruminant and poultry farmers on the safe storage and disposal of poultry litter and the proper preparation of silage are still needed to prevent further cattle botulism outbreaks [Reference Otter37].
The grass silage was not initially suspected as a potential vehicle of C. botulinum or its toxin, as the senior heifers were not affected, despite being fed this silage, until almost a month after the onset of the outbreak. The difference in botulism development could be due to the differences in feed distribution between milking cows and heifers. The feed was mixed manually for heifers, which could more easily sort and refuse the contaminated silage, as observed by the farmer. Using a mixer may have minimized the poor palatability of clostridial-contaminated silage and made it more difficult to sort the ingredients, thus resulting in the ingestion of the contaminated silage [Reference Lindström9]. Another possibility could be that only some of the silage was contaminated and distributed to milking cows, while the rest was uncontaminated or only slightly contaminated and given to the heifers. Finally, the feed mixer could also be contaminated by a spoiled silage pocket or a carcass. However, in this case, we should not have found C. botulinum in the silage collected from the bunker silo at the end of the outbreak.
Some authors have hypothesized that cattle exposed to BoNTs were also most likely exposed to spores of C. botulinum, and may thus have developed toxicoinfection caused by C. botulinum colonization of the GI tract and in situ germination and toxin production [21, Reference Carlier and Popoff32]. This form of botulism has been reported in human infants and foals, but should not affect adult vertebrates, in which normal intestinal bacteria compete with the germination and growth of BoNT-producing clostridia [Reference Critchley2]. A form of toxicoinfection in adult cattle has been described in Germany and called ‘visceral botulism’. However, the implication of BoNT-producing clostridia in such a case is still debated [Reference Böhnel, Schwagerick and Gessler38, Reference Seyboldt39]. Toxicoinfection has also been suggested to explain longer incubation periods in biphasic botulism outbreaks in cattle [Reference Neill, McLoughlin and McIlroy40]. Under this scenario, animals that ingested a smaller amount of BoNTs would be temporarily debilitated, with altered GI tract motility, providing conditions that allow C. botulinum growth and secondary toxicoinfection. In the present outbreak, almost all deaths occurred within 19 days after ingestion of the contaminated grass silage, which is coherent with the incubation period previously described of 24 h to 17 days [Reference Deprez, Mainil, Duschesnes, Granum and Menozzi6, Reference Anniballi41]. The death that occurred on day 75, 36 days after the last ingestion of the contaminated silage, could be explained by a secondary toxicoinfection as described by Neill et al. [Reference Neill, McLoughlin and McIlroy40]. The last death concerned a cow that had never been fed with the contaminated grass silage. Despite the farmer having reported clinical signs compatible with botulism (weakness of the hind limbs, progressively leading to lateral and sternal recumbency), this case occurred in early lactation and could also be due to other diseases, notably milk fever.
CONCLUSION
Type D bovine botulism outbreaks linked to contact with or close proximity to poultry litter are increasingly reported in Western Europe. Until now, the presence of carcasses of birds that died during production was regarded as the likely source of BoNTs. In this study, the epidemiological and laboratory investigation suggests another contamination scenario, with a stack of poultry litter as the source of C. botulinum spores and insufficiently acidified grass silage as the medium supporting growth and toxin production from C. botulinum spores. As poultry carcasses were collected and removed from the poultry barn daily, it seems unlikely that the source of toxins or spores in poultry litter was poultry carcasses. Asymptomatic carriage of type D/C C. botulinum organisms by poultry in their gut should be further investigated. These findings highlight the fact that precautions should be taken for the safe disposal of poultry litter and reinforce the importance of proper ensiling of feed materials to prevent bovine botulism.
ACKNOWLEDGEMENTS
The authors acknowledge the farmers, Dr Maertens (veterinary practitioner, Le Teilleul, France) and Dr Leboeuf (GDS 50, Saint-Lô, France) for their warm welcome and contributions. This research received no specific grant from any funding agency in the commercial or not-for-profit sectors.
DECLARATION OF INTEREST
None.
ETHICAL STANDARDS
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.








