INTRODUCTION
Foodborne disease is an important cause of human infectious intestinal disease (IID) which costs an estimated £0·75 billion per annum [Reference Humphrey1] to the UK. In 2000, the newly created Food Standards Agency (FSA) set a target of reducing the incidence of foodborne disease by 20% by 2006. It identified five important pathogens to target – Campylobacter, Salmonella, C. perfringens, Escherichia coli O157 and Listeria monocytogenes. Provisional figures from 2004 data indicate a reduction of 18·7% with a significant contribution attributed to falling numbers of cases of campylobacteriosis [Reference Bell2], an organism which tends to be associated with white rather than red meat. In contrast, verocytotoxigenic E. coli (VTEC) O157 has increased in importance in recent years and although low numbers of cases are reported in comparison with Salmonella and Campylobacter, this organism is associated with serious sequelae, high mortality rates and a significant economic burden [3–Reference Adak, Long and O‘Brien5].
C. perfringens food poisoning is a relatively mild form of food poisoning. This organism is widespread and it was not therefore considered a prudent use of resources to investigate its carriage. Proper disinfection of surfaces used in food production is the most efficient way of controlling the problem of C. perfringens food poisoning [Reference Brynestad and Granum6]. Listeria monocytogenes was not included in the present study as it is associated with chilled products such as commercially prepared salads and cooked meats.
A previous abattoir study had looked at the prevalence of Yersinia enterocolitica, which is common in livestock. As a cause of human gastroenteritis, it is probably underestimated in the UK [Reference McNally7] and this survey allowed a comparison with previous data in terms of prevalence. In addition comparisons could be made between the survey samples and human outbreak biotypes.
Recent surveillance of foodborne outbreaks of IID by the Health Protection Agency (HPA) indicate that 16% of VTEC O157 outbreaks were associated with the consumption of red meat [Reference Smerdon8] and that 85% were considered to be foodborne [Reference Adak, Long and O‘Brien5]. In order to reduce morbidity and mortality associated with such outbreaks, there is a need to monitor the food chain and identify risk factors thereby allowing effective control measures to be developed.
A 12-month abattoir survey was designed to collect samples of intestinal material from cattle, sheep and pigs in GB intended for human consumption [Reference Milnes9]. In addition to collecting ruminant rectal and porcine caecal samples, data relating to the originating animal were collected to provide epidemiological information about the slaughter population. The samples were examined for VTEC O157, Salmonella, thermophilic Campylobacter and Y. enterocolitica. The primary objective of the study was to provide estimates of the prevalence of carriage of these organisms and this work has been published [Reference Milnes10]. In addition, the information collected was used to investigate potential risk factors and it is this data which is reported in the present study.
MATERIALS AND METHODS
Sample collection
A detailed description of the 2003 abattoir survey, collection and sampling of intestinal contents and microbiological examination are reported in detail elsewhere [Reference Milnes10]. Sample-size estimates were undertaken based on the prevalence estimates by previous similar studies [Reference Davies11, Reference Paiba12]. The samples sizes generated were designed for estimating the prevalence of carriage and not for assessing differences in risk groups.
The target population was cattle, sheep and pigs entering the human food chain. A total of 327 red-meat abattoirs in GB were contacted and invited to participate. These included abattoirs that slaughtered cattle aged <30 months (thereby eligible to enter the food chain); sheep and pigs. The number of samples collected from each abattoir was proportional to its throughput and if <2 samples were calculated for the 12-month study period then the abattoir was excluded. Therefore, although 144 abattoirs agreed to participate, 51 were subsequently excluded due to low throughput.
All samples were examined for VTEC O157 using the methodology employed by a previous abattoir study [Reference Paiba12]. E. coli O157 isolates were examined by polymerase chain reaction (PCR) for the presence of the verocytotoxin genes VT1 and VT2 and the intimin eae gene in order to identify the isolate as VTEC O157. As a consequence, both E. coli O157 and VTEC O157 isolates were identified.
All ruminant samples were tested for Salmonella with 25% additionally examined for thermophilic Campylobacter and Y. enterocolitica. All pig samples were examined for Y. enterocolitica with 25% additionally examined for Salmonella, and Campylobacter. Bacteriological examinations were performed based predominantly on methodologies used in previous surveys [Reference Paiba and Gibbens13].
Data collection
All cattle born in or imported to GB since 1 July 1998 must have a cattle passport which is issued by the British Cattle Movement Service (BCMS). All movements are recorded on this document as well being reported directly to the BCMS. The carcases of animals which do not have correctly completed passports are not allowed to enter the food chain. Each batch of cattle and sheep moved in GB must have a record kept of the movement, which includes the species, number of animals, the date of movement and the addresses of the points of departure and arrival. These movement details are captured on a form as part of the Animal Movements Licensing System (AMLS). Abattoir records were used to obtain additional information about animals including slaughter weight information. The on-site information was extremely useful for pigs. Information was collected from the abattoir about the size of the day's kill and the type of abattoir – single or multi species.
For each sample, information relating to the animal of origin was collected from cattle passports, AMLS forms and Meat Hygiene Service (MHS) ante-mortem sheets:
• Date of birth (cattle) or approximate age for sheep (using dentition) or pigs.
• Sex.
• Breed (cattle only).
• Whether the last premise of domicile was the farm of birth.
• County of the source farm, which were then geographically identified as areas – North East, North West, South East and South West.
• Whether the animal has come to slaughter direct from the farm, or via a market.
• Movement history – distance and time of journey to abattoir (all species), and number of movements in previous 30 days (cattle only).
• Abattoir information – day's kill, time spent in lairage, date of arrival, date of slaughter, and whether the animal had been fed or given bedding.
The sample and information relating to the originating animal were identified using a unique barcode for each animal, which was attached to both the submission form and the retrieved sample. This allowed for anonymity of the origin of the sample. In the laboratory the sample and test results were collated using the VLA Sample Management System, which tracks the progress of the sample from receipt to the reporting of results.
Analysis
Data from the submission forms were entered onto the VLA Sample Management System at the receiving laboratory, with distance travelled entered centrally at VLA Weybridge. The data were checked for errors by rechecking 5% of submissions forms and 2·5% of distance travelled information. The data were then transferred to an Access database and frequencies and cross-tabulations were run to check for inconsistencies and implausible values. The data were then rechecked and submissions with implausible values removed from the analysis.
Descriptive univariable analysis was undertaken in Access and Stata version 8 (StataCorp, USA). Many of the variables had skewed distributions and therefore the median rather than the mean is given as a summary statistic.
Univariable logistic regression models using outcome for each pathogen as the dependent variable were used to screen for potential risk factors. In the case of Campylobacter, the outcome was either C. coli or C. jejuni as these species are considered the primary zoonotic species.
Where there were sufficient positive samples, multivariable logistic regression models were fitted to the outcomes with age, distance, lairage time and transport time treated as either continuous or categorical explanatory variables using quartiles.
Region of origin and season were included in all models as potential confounders because it was thought that either or both might be associated independently with the outcomes and potential risk factors. A combination of forward and backward stepwise logistic regression procedures was used to identify variables that were associated with the presence of infection. These main-effects models included all variables that were significant at P<0·05 by a likelihood test. The final models included in addition any interactions significant at P<0·05 between the main effects selected in addition to region and season of origin. Akaike's Information Criterion (AIC) was used to choose between the categorical and continuous forms of the variables. Goodness of fit was assessed by the Hosmer–Lemeshow test. Where appropriate, the final models were re-estimated with an adjustment for the clustering of samples by submission.
RESULTS
Characteristics of sampled population
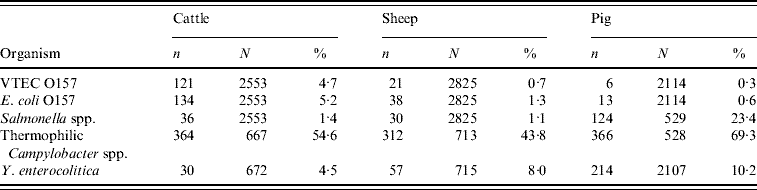
Of the 93 participating abattoirs, 28 (30%) slaughtered single species and 65 (70%) were multi-species abattoirs. During the 12-month study a total of 7492 intestinal samples were collected from 93 abattoirs in GB. They represented 2553 cattle rectal samples, 2825 sheep rectal samples and 2114 pig caecal samples. The number of isolates by host species is shown in Table 1.
Table 1. Isolates of foodborne pathogens by host species
The number of samples collected from the abattoirs was proportional to throughput and therefore varied through the year with the minimum in April and the maximum in October.
The day's kill ranged from 5–1600 cattle (median 180); 29–6500 sheep (median 2000) and 34–3100 pigs (median 1750). The age of sampled cattle ranged from 2 to 30 months (median 24 months) and the age range of sampled pigs was 4–36 months (median 6 months). Most lambs (71%), were born within the current year's lambing season and were therefore aged <1 year at slaughter.
The three most common cattle breeds presented were Limousin cross (28%), Charolais cross (20%) and Simmental cross (10%) The most frequently presented dairy animal was the Holstein Friesian representing 6·5% of the slaughter population. Beef breeds predominated accounting for 91% (2314/2535) of all samples. Within this group 84% were Continental beef breeds with only 16% British breeds.
The production purpose of submitted pigs was bacon (71%), pork (25%) and cull (3%). The mean weight of the sampled carcases was 78·7 kg with a range of 28·5–242·5 kg.
Movement history
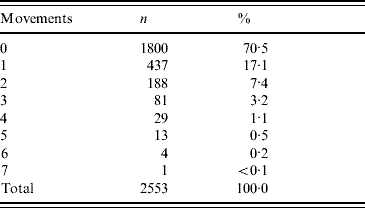
Using the cattle passports, farm of birth and any subsequent movements were reviewed, which showed that 32% came directly from their farm of birth. On 88% of occasions, the animal came from a farm with 12% being sourced from market. In the 30 days prior to slaughter, 70% were moved directly from their source (farm or market) to the abattoir. Table 2 reviews the movement history.
Table 2. Cattle movements in the 30 days prior to slaughter excluding final journey to abattoir
Transport time ranged from 0·5 to 24·5 h with a median of 2 h. The distance travelled by the cattle to the abattoir ranged from 9 to 649 km (median 69 km). For sheep and pigs, respectively, the time spent in transit to the abattoir ranged from 0·5 to 46 h and 0·5 to 23 h (median for both 2 h) with the distance ranging from 9 to 779 km for sheep and 9 to 879 km for pigs (median 69 km for both ).
Lairage information
The time the animal was held in lairage ranged from 0 to 48 h (median 11 h) for cattle; 0–47 h (median 3 h) for sheep and 0–48 h (median 14 h) for pigs. This is illustrated by the bimodal distribution seen in Figure 1.
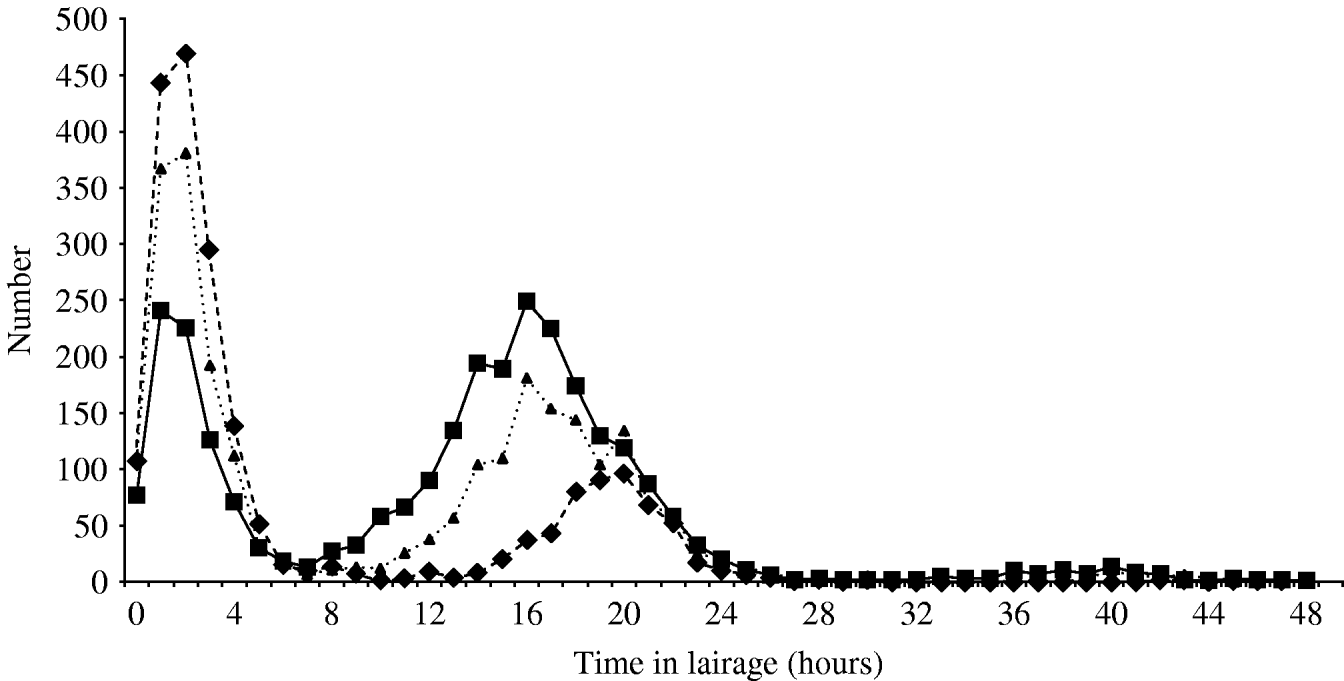
Fig. 1. Time spent in lairage by cattle (····▴····), sheep (––▪––) and pigs (- -◆- -).
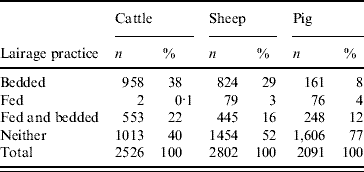
Within the lairage, 40% (1013/2526) of cattle were not fed or given bedding. Of the remainder, 38% were bedded, 0·1% were only fed and 22% were both fed and bedded. Many were not fed for periods >12 h. Thirty-nine per cent of sheep which had spent >12 h in lairage had been neither fed nor bedded. In total, only 120 sheep were reportedly kept in lairage for >24 h. Most pigs were neither fed nor given bedding in the lairage. The lairage practices for each species are tabulated in Table 3.
Table 3. Lairage practices for cattle, sheep and pigs in 2003 abattoir study
Statistical analysis
Multivariable techniques were used for the cattle data but not for some of the other organism/species combinations due to the small number of positive samples.
VTEC O157
The final model for VTEC O157 carriage in cattle consisted of three variables – the two confounding variables of region and season, and age in months (Table 4). The summer period, June–August, was associated with an increased risk of carriage compared to the September-November period [odds ratio (OR) 2·0, 95% confidence interval (CI) 1·21–3·31]. As the age of the sampled animal increased, VTEC O157 was less likely to be cultured (OR 0·96, 95% CI 0·93–0·99).
Table 4. Logistic regression model of variables associated with carriage of VTEC O157 in cattle (n=2535)
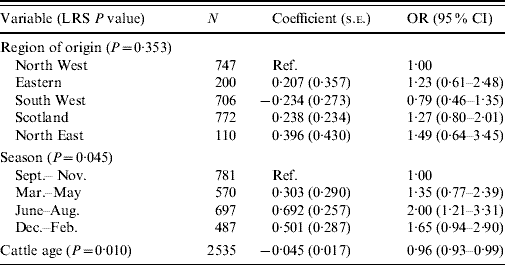
LRS, Likelihood ratio statistic; OR, odds ratio; CI, confidence interval.
LR χ2 (8 d.f.)=21·08, P=0·007.
Hosmer–Lemeshow test: χ2 (8 d.f.)=6·65, P=0·574.
Only bivariate analysis was undertaken for sheep and pigs due to the small number of isolates. Selected results – region of origin, season and other variables with P<0·10 for sheep are shown in Table 5. VTEC O157 was more likely in animals aged >6 months (OR 6·7). The region of the abattoir also appeared influential with abattoirs in the Eastern part of the country more likely to produce positive results (OR 5·75, 95% CI 1·8–18·40).
Table 5. Association between variables of interest and outcome of VTEC O157 carriage in sheep
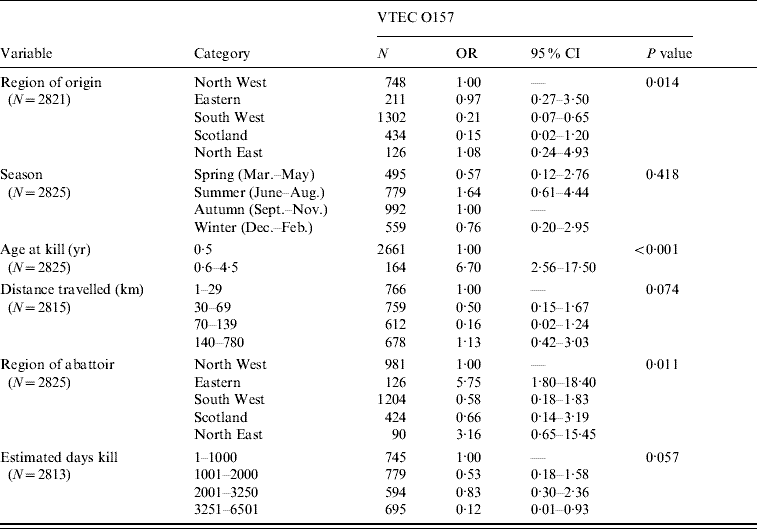
OR, Odds ratio; CI, confidence interval.
There were only six isolates of VTEC O157 from pigs and no association between variables and outcome was seen.
Salmonella
Cattle
There were 36 isolates of Salmonella from cattle and the results from the final model are shown in Table 6. These results indicate associations with region of origin, season, breed and day's kill. From this analysis the Eastern region, dairy cattle and the summer period appear to produce the greatest risk of Salmonella carriage. The risk was lowest in abattoirs with the greatest throughput.
Table 6. Logistic regression model of variable associated with Salmonella in cattle (n=2405)
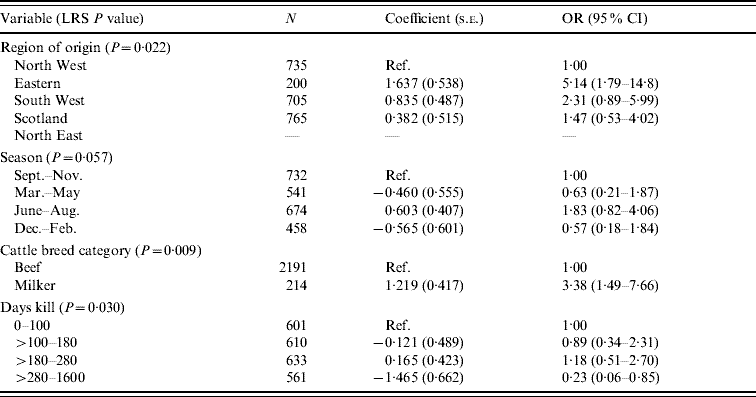
LRS, Likelihood ratio statistic; OR, odds ratio; CI, confidence interval.
LR χ2 (10 d.f.)=30·38, P<0·001.
Hosmer–Lemeshow test: χ2 (8 d.f.)=4·99, P=0·759.
Sheep
A multivariable model was not possible for sheep data due to the small number of isolates. Table 7 shows the results from bivariate analysis. A regional association was found with abattoirs in, and animals from, the North East most likely to carry the organism. Seasonality was shown with spring the period of highest risk. Age appeared to be a risk factor with carriage more likely in animals aged >6 months. Surprisingly, lairage times of <4 h were associated with carriage and high-throughput abattoirs appeared protective. Stratifying by region, did not provide evidence of an interaction between the region and these significant variables (lairage times and throughput) or of increased odds ratios within the North East region. Despite this, the North East area had abattoirs with the lowest average daily kill.
Table 7. Association between variables of interest and outcome of Salmonella in sheep

OR, Odds ratio; CI, confidence interval.
Pigs
An initial model was run for the pig data and is tabulated in Table 8. The variables region of origin, time in lairage and purpose were associated with the outcome. The North Eastern region had the highest risk of producing pigs with intestinal Salmonella carriage. Holding pigs in lairage for >12 h also increased the risk of carriage. There is no clear risk associated with purpose, but there is some indication that cull sows, which are likely to be older breeding animals were less likely to carry Salmonella than finishers. In addition, there was evidence of an interaction between feeding and bedding in the lairage. Individually, feeding and bedding had no effect on the outcome, but not feeding when there was no bedding increased the risk of Salmonella carriage relative to not feeding when bedding was provided (OR 4·4, 95% CI 1·01–18·8).
Table 8. Logistic regression model of variables associated with Salmonella in pigs, using lairage time as a variable (pigs Salmonella, n=520)
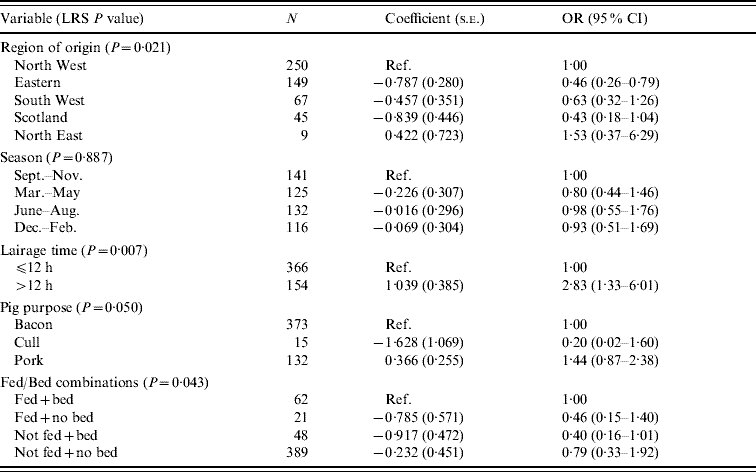
LRS, Likelihood ratio statistic; OR, odds ratio; CI, confidence interval.
LR χ2 (13 d.f.)=41·16, P<0·001.
Hosmer–Lemeshow test: χ2 (8 d.f.)=9·54, P=0·299.
The effect of time in lairage as a risk factor was investigated further by replacing the variable ‘lairage time’ with ‘day of kill’ which represented whether the animal was killed on the day it arrived at the abattoir. This gave similar findings to the previous model and reinforces the effect of time in lairage. When a pig is not killed on its day of arrival at the plant, then the risk of Salmonella carriage increases significantly (OR 3·3, 95% CI 1·5–7·0).
Campylobacter
Cattle
Carriage of C. jejuni and C. coli was not associated with either region or season. However, cattle slaughtered in facilities used for multi-species were less likely to carry these species of Campylobacter than those killed in dedicated cattle abattoirs (OR 0·58, 95% CI 0·37–0·90, P=0·015) and carriage was less common in older animals (OR 0·96, 95% CI 0·93–0·99, P=0·04).
Sheep
There was an association of thermophilic Campylobacter spp. with season (P=0·023), the winter months a period of maximum carriage. Management practices in the lairage were also relevant with feeding a risk factor (OR 2·27, 95% CI 1·45–3·57, P<0·001) and bedding a protective factor (OR 0·60, 95% CI 0·41–0·88, P=0·008).
Pigs
The only variable associated with C. coli and C. jejuni carriage was time spent in lairage. A lairage time of <12 h was a risk factor (OR 1·72, 95% CI 1·16–2·56, P=0·007). When the model was rerun, replacing lairage time with the variable ‘day of kill’, a similar effect was seen. Thus killing the animal on the day that it arrived was confirmed as a risk factor.
Y. enterocolitica
Cattle
The carriage of Y. enterocolitica in cattle was strongly influenced by season (Table 9). An increased risk is seen in the 6 months from December to May. Age is also shown to influence the outcome with an increased risk in older cattle.
Table 9. Logistic regression model of variables associated with Yersinia enterocolitica in cattle (n=668)
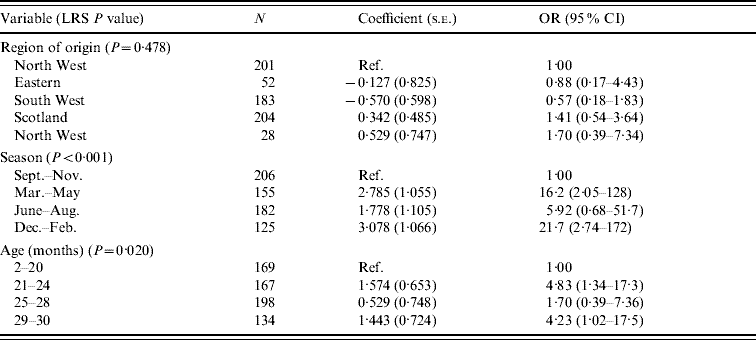
LRS, Likelihood ratio statistic; OR, odds ratio; CI, confidence interval.
LR χ2 (10 d.f.)=36·58, P<0·001.
Hosmer–Lemeshow test: χ2 (8 d.f.)=4·53, P=0·806.
Sheep
For sheep both season and feeding within the lairage were shown to be influential variables. The 6-month period from December to May had a higher risk than the subsequent summer and autumn periods. Feeding within the lairage was also shown to be a risk factor (OR 2·00, 95% CI 1·03–3·85, P=0·048).
Pigs
For pigs, as with the ruminants, the 6-month period from December to May was shown to be the highest risk period for carriage of Y. enterocolitica (Table 10). Holding the animal overnight at the abattoir was also a risk factor as animals which were killed after their arrival date had an increased risk of carriage. There was a positive association with distance but the effect appeared to be minimal.
Table 10. Logistic regression model of variables associated with Yersinia enterocolitica carriage in pigs (n=2080)
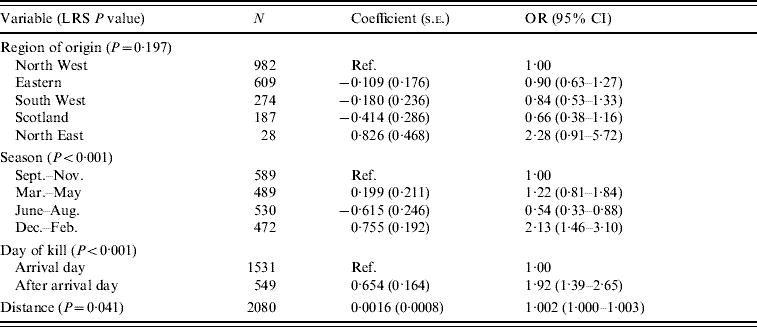
LRS, Likelihood ratio statistic; OR, odds ratio; CI, confidence interval.
LR χ2 (9 d.f.)=70·47, P<0·001.
Hosmer–Lemeshow test: χ2 (8 d.f.)=8·11, P=0·423.
DISCUSSION
Study population
Data relating to individual cattle, sheep and pigs at slaughter in GB in 2003 were analysed in order to describe the population that had been sampled and to investigate risk factors for the intestinal carriage of VTEC O157, Salmonella, C. jejuni/coli or Y. enterocolitica.
Information relating to cattle samples proved relatively easy to collect due to the availability of cattle passports and the necessity for such documentation to be provided by all cattle at the abattoir. Comparison of the available information on sampled animals with national slaughter populations showed that the two populations were similar and estimated to represent a large proportion of the slaughter population (63% of cattle, 58% of sheep and 86% of pigs) [Reference Milnes10]. With reference to cattle very similar proportions of breeds were slaughtered during 2003 as seen by CTS data in RADAR (Rapid Analysis and Detection of Animal-related Risk system). This provides some assurance that the sampled population was representative of the target population – animals entering the human food chain.
Transport and lairage details
Reviewing the movement history of cattle revealed that 30% moved between units at least once in the month prior to slaughter. Due to the 6-day standstill [14] only five movements should be possible, but exemptions are possible for breeding animals. In fact, only five (0·2%) cattle had more than five movements and it is not known whether these animals could have been subject to exemptions or whether there had been a transcription error.
Transport times for the different species were similar and averaged between 2 and 3 h. Times are influenced by the proximity to an abattoir and by The Welfare of Animals (Transport) Order 1997 [15] which promotes shorter journey times by enforcing rest times during journeys. These regulations state that, for ruminants, after a period of 14 h transport a rest period of 1 h should be given before allowing transport for a further 14 h. There were two sheep within the sampled population where the journey time exceeded 29 h. However, the general observation of this study is that the Order is adhered to by transporters.
In all species, lairage times showed a bimodal distribution with two peaks – one shortly after arrival and a second around 18 h indicative of animals held overnight. This pattern is noted in other abattoirs (R. Milne, MLC, personal communication). Regulations state that animals kept in lairage for >12 h should be fed [16], but the data from this survey indicate that this did not always occur. It is possible that the question asked was misinterpreted. However, the practicalities of feeding animals within the lairage, together with the increased costs and the preference for an empty intestine suggest that the data are likely to be correct.
Study results
The study was designed primarily to estimate the prevalence of intestinal carriage of various foodborne pathogens in red-meat animals. Although sample sizes were adequate for this, the relatively low prevalence of various organisms meant that multivariable analysis was not possible for all species and all pathogens investigated. All samples and the farms of origin were anonymous, therefore important influential factors could not be included. However, the abattoir is an important site in the food chain and any identified risks associated with the management of animals around this period may have potentially significant public health effects.
VTEC O157
Multivariable analysis for VTEC O157 carriage was only possible for cattle due to a low prevalence in pigs and sheep. Indeed there were only two significant variables – season and age in the final model. The warmer summer period was associated with a higher risk of VTEC O157, a pattern that has been reported by previous researchers [Reference Paiba12, Reference Hancock17–Reference Bonardi21]. However, Scottish researchers have also shown an autumnal rise, which they linked to housing [Reference Ogden, MacRae and Strachan22, Reference Synge23]. The present study also identified a second peak during the late autumn/early winter months [Reference Milnes9].
In our study, the age of the investigated population was restricted to slaughter cattle and ranged from 2 to 30 months. The data were negatively skewed with a median age of 24 months. Despite this, age was shown to be significant and protective against VTEC O157 carriage, agreeing with other researchers [Reference Hancock17, Reference Van Donkergoed, Graham and Gannon20, Reference Paiba24]. Paiba and colleagues [Reference Paiba24] sampled 4663 cattle of varying ages from 75 farms, where they found that infection was highest in calves aged between 2 and 6 months and declined thereafter. However, Italian studies of slaughter cattle found similar proportions in both feedlot and older dairy cull cows with no isolates from veal calves [Reference Bonardi21, Reference Conedera25]. It was proposed that the low pH of the abomasum in pre-weaned calves inhibits survival of the organism compared to the less acidic environment of the adult rumen.
Previous research has reported that transport and fasting may increase shedding of VTEC O157, perhaps due to changes in rumenal pH [Reference Rasmussen26]. However, in the present study no clear association between journey time and faecal carriage was found. There was an increase in odds ratio between cattle transported for <90 min and those transported between 90 min and 2 h. After this time, the odds ratios declined. These time periods are narrow due to the spread of data which may have made it difficult to demonstrate an effect. But over all four quartiles it would appear that transport time does not affect VTEC O157 carriage, a finding agreeing with a Canadian study which found no association with VTEC carriage and distance travelled to the abattoir [Reference Van Donkergoed, Graham and Gannon20].
Salmonella
Nearly a quarter of pigs carry Salmonella, which is a cause for concern. Attempts are being made through the UK Zoonoses Action Plan (ZAP) [Reference Misselwitz27] to monitor and reduce the organism in pig herds. With awareness and knowledge of the prevalence and risk that this creates for the food chain, the abattoir is an important stage where infection can potentially be reduced or amplified. The findings of this study, support other work showing that increased lairage times are a risk factor for isolation of Salmonella [Reference Morgan, Krautil and Craven28]. The present study addresses only caecal colonization and did not examine carcase contamination, which more closely reflects public health risk. Studies have shown that pigs can rapidly acquire infection in periods as short as 2 h after contact with a contamination source [Reference Hurd29], with three sources of infection identified: the farm of origin, transportation and the lairage [Reference Vieira-Pinto, Tenreiro and Martins30]. Therefore holding pens which may already be contaminated and the presence of infected pigs in a stressful environment could allow amplification of Salmonella carriage. An American study comparing Salmonella isolation between pigs killed on farm and after transport and lairage, reported a significantly higher carriage in pigs sampled at the abattoir, and concluded that holding pens were an important Salmonella control point [Reference Hurd31].
Pigs, from the North East region of GB appeared to be most associated with positive results, a finding which has also been highlighted by immunosurveillance results from the ZAP scheme (M. E. Clough et al., unpublished observations). A strong interaction between feeding and bedding was shown, with pigs which had not been fed or given bedding contributing an increased risk. This observation may refer to management practices and may indicate a more stressful environment and a greater likelihood of ingestion of contaminated material from the pen.
Geographical variability was also shown in Salmonella carriage in cattle, with those from the Eastern region most likely to be carriers. However, another recent study of dairy farms identified the South West as an area of higher risk. This study looked only at dairy farms and identified that the effect may be biased by a high dairy farm density in the area [Reference Davison32].
Milking cattle were shown in this study to be at a higher risk of Salmonella carriage than beef cattle. Whether this is a breed or management effect could not be determined. It is plausible for dairy herds to have a higher prevalence of Salmonella due to a more intensive management system. Unknown farm-associated factors may also be related to these differences. Stress is known to predispose animals to disease and dairy cattle in early lactation [Reference Fossler33, Reference Fitzgerald34]; large herd size and diet [Reference Davison32, Reference Kabagambe35] have all been associated with increased shedding of Salmonella [Reference Wells36, Reference Huston37].
Seasonality was implicated and the summer period shown to be a risk period. This agrees with previous research both in the UK and North America [Reference Fossler33, Reference Evans and Davies38] and suggests an association with cattle at grass having access to contaminated pasture through muck spreading, wildlife sources or flooding. In sheep a seasonal effect was also shown with the spring period associated with greatest carriage. This would reflect the lambing period as a time of greatest risk of within flock carriage.
Campylobacter
No consistent seasonal pattern was shown for Campylobacter carriage in the livestock species investigated. This agrees with the previous abattoir survey in 1999/2000. However, in contrast, a late spring/early summer peak in human cases of campylobacteriosis is consistently recorded in England and Wales [39, Reference Louis40]. Louis and colleagues [Reference Louis40] found that this human seasonal pattern was temperature driven with most marked seasonality found in young children. Similar patterns are recorded in many temperate countries [Reference Nylen41, Reference Stanley and Jones42] and it seems likely that seasonality is linked to environmental factors rather than foodborne sources.
Campylobacter jejuni/coli colonization in cattle, sheep and pigs is usually asymptomatic. However, these organisms can cause congenital infections in both cattle and sheep, usually resulting in abortion. Within a few months of life, infected calves will shed large numbers of organisms with excretion decreasing by age 7 months. Previous research has shown that adult cattle are less likely to be infected than calves [Reference Adak43–Reference Giacoboni45]. In fact, an age effect was demonstrated by the present study despite the fact that the population was restricted to slaughter-aged cattle.
Interestingly, an increase in carriage of Campylobacter in sheep was found during December–February. Jones et al. [Reference Jones, Howard and Wallace46] reported intermittent shedding depending on the season with highest numbers coinciding with periods of increased stress, e.g. lambing, weaning or movement. Indeed a spring peak in slaughter lambs was also reported by Stanley et al. [Reference Stanley47]. Most sheep in our survey were aged <12 months and so lambing period stress would not be applicable; however, this may reflect the generally higher number of organisms in the ovine environment at this time.
Lairage practices, particularly not providing bedding but feeding, influenced the carriage of Campylobacter in sheep but not in pigs or cattle. It may be speculated that this was due to changes in gut flora associated with a period of feed deprivation followed by access to feed. Such changes may reflect increased stress mediated by lairage practices.
For pigs, a surprising finding was that an increased lairage time was found to be protective. However, an abattoir study by Warriss et al. [Reference Warriss48] reported that longer lairage time reduced stress levels based on the concentration of cortisol, lactate and creatine phosphokinase in the blood.
Y. enterocolitica
For all three species, season was found to be significantly associated with carriage of Y. enterocolitica. The winter period, December–February was the period of most risk. A similar seasonal pattern has been reported by the 1999/2000 pig abattoir survey [Reference McNally7, Reference Barkocy-Gallagher49], where carriage was highest during December and January with carriage significantly reduced during the summer months. Seasonality appears to play an important role in human disease with a higher number of cases found during the cooler months. Pigs are thought to be reservoirs for some human infections in GB, particularly with BT3(O:9) [Reference Morgan, Krautil and Craven28] and the seasonal trends seen may correspond with factors related to increased pig meat consumption. For example, surveillance from five Foodborne Disease Active Surveillance Network (FoodNet) sites in the United States reported a seasonal peak in December only in black persons. It was hypothesized that this was related to the consumption of the pork product ‘chitterlings’ which is consumed by this minority group at that time of year [Reference Ray50].
CONCLUSIONS
Investigation of risk factors was hampered by the limitation of the dataset as on-farm factors were not accessible and, in some cases, the low level of carriage. However, in many cases the data support previous research and give an insight into factors which would influence carriage.
The abattoir and lairage are critical control points where manipulation of the production process could have serious consequences for carcase contamination levels. The results from the present study highlight areas where future research is warranted to identify occasions where changes in management practices may influence the carriage of common foodborne bacterial pathogens by animals entering the food chain. Not all the findings of the study appear biologically plausible and in many areas the study highlights areas where further research may prove rewarding rather than attempting to give definitive guidelines for abattoir practices.
However, many findings do appear credible and support other related work. For example, the implication that a reduction in lairage time for pigs would reduce Salmonella carriage at slaughter. Salmonella carriage in pigs is an issue in GB and the risk factors for the end product through carriage, amplification of infection are important.
Although a reduction in carriage in food-producing animals is actively sought to protect the consumer, eradication of ubiquitous organisms by a subclinical host is unlikely to be achievable Therefore, reducing the pathogen burden by identifying those factors which influence carriage will contribute to reducing the risk to human health as part of a complete food chain strategy.
ACKNOWLEDGEMENTS
The authors thank the abattoirs and their staff for participating in this study. We gratefully acknowledge the staff of the Meat and Livestock Commission for sample collection and data, with particular reference to Ron Milne for coordinating this work. The VLA Regional Laboratory staff are gratefully acknowledged for their assistance in both sample and data collection and the laboratory examination of samples. Kathy Speed and Katherine Sprigings are thanked for their assistance. We are also grateful to the staff of the Health Protection Agency Colindale for phage typing of VTEC O157 and serotyping of Yersinia isolates. This project was funded by the Department for Environment, Food and Rural Affairs (Project FZ2009).
DECLARATION OF INTEREST
None.















