INTRODUCTION
Rotavirus is the most common cause of gastroenteritis in children aged <5 years. Recent evidence suggests that it is responsible for 48% of all cases of gastroenteritis in the community in the United Kingdom [Reference Iturriza Gomara1], and that this results in an economic burden of some £11.5 million [Reference Lorgelly2]. A recent review of the global prevalence of rotavirus found that by the age of 5 years, nearly every child will have experienced an episode of rotavirus gastroenteritis; as a result one in five will seek the advice of a health professional, one in 65 will be hospitalized, and approximately one in 293 will die [Reference Parashar3]. The burden is greatest in developing countries, and as such the World Health Organization regards the development of a safe and effective vaccine against rotavirus infection a high priority for improving global health.
Since identification of the rotavirus particle and the recognition that it can cause severe illness, researchers have sought to develop a vaccine which would prevent or reduce the associated morbidity and mortality. In 1998 such a vaccine, the tetravalent rhesus-human reassortant rotavirus vaccine [Rotashield® (RRV-TV)] (Wyeth Laboratories Inc., Marietta, PA, USA), was approved for use in the United States. Two economic evaluations of this vaccine were undertaken, both of which suggested that the vaccine had the potential to be cost effective [Reference Smith4, Reference Tucker5]. However, a year after it was introduced the vaccine was withdrawn due to reports of a possible association between the vaccine and intussusception (the telescoping of one portion of the intestine into another).
Subsequent research has now resulted in the development of two new vaccines [Reference Vesikari, Giaquinto and Huppertz6]. The first, Rotarix® (GlaxoSmithKline Biologicals, Rixensart, Belgium), is a live attenuated monovalent vaccine containing human rotavirus strain RIX4414 of G1P1A P[8] [7]. It is administered to infants orally at ages 2 and 4 months. The second, Rotateq® (Merck & Co., Inc., West Point, PA, USA), is a live pentavalent reassortant vaccine containing bovine rotavirus and surface proteins of human serotypes G1, G2, G3, G4, and P1A [Reference Vesikari8]. It is also an oral vaccine, which requires three doses between ages 2 and 8 months. To date Rotateq has been approved by the FDA, in the United States and by Health Canada and recently received approval from the European Medicines Agency (EMEA). Rotarix also has similar European approval, which means that negotiations with individual countries within Europe can begin.
With the approval from the EMEA, individual countries within Europe will be reviewing information on each vaccines' safety and efficacy and may also consider the cost effectiveness of vaccination. To date four economic evaluations have been undertaken which examine the cost effectiveness of a rotavirus immunization programme; the two described above which were undertaken with respect to the US population [Reference Smith4, Reference Tucker5], one within an Australian context [Reference Carlin9] and a recent evaluation from Uzbekistan [Reference Isakbaeva10]. However, as the paper by Carlin et al. [Reference Carlin9] argues: ‘decisions about cost effectiveness of preventative programmes are highly sensitive to local factors such as incidence, rates of hospitalization, costs of hospital care and costs of vaccine delivery systems’, therefore, it is necessary to undertake an economic evaluation within the UK context. Furthermore, since the majority of these evaluations were published there has been a vast amount of research into the disease and its burden and as such this evaluation will update many of the estimates that were previously used.
This paper reports the results of a cost-effectiveness analysis of introducing a rotavirus immunization programme into the national immunization schedule in the United Kingdom. A decision analytical model is used which combines probability and cost estimates of the various consequences and outcomes of immunization and exposure to the disease. The analysis is performed from both the societal and health sector perspectives, and the sensitivity of the results to different parameters and threshold values is also examined.
METHODS
The decision model
A decision tree was constructed to compare a universal vaccination programme (that is one that would be included in the national immunization schedule) with no programme (current practice). Figure 1 displays the tree, which draws heavily on the earlier work of Smith et al. [Reference Smith4], Tucker et al. [Reference Tucker5] and Carlin et al. [Reference Carlin9]. Calculations and analyses were undertaken using the decision analysis software TreeAge Pro, version 8.1 (TreeAge Software, Williamstown, MA, USA).
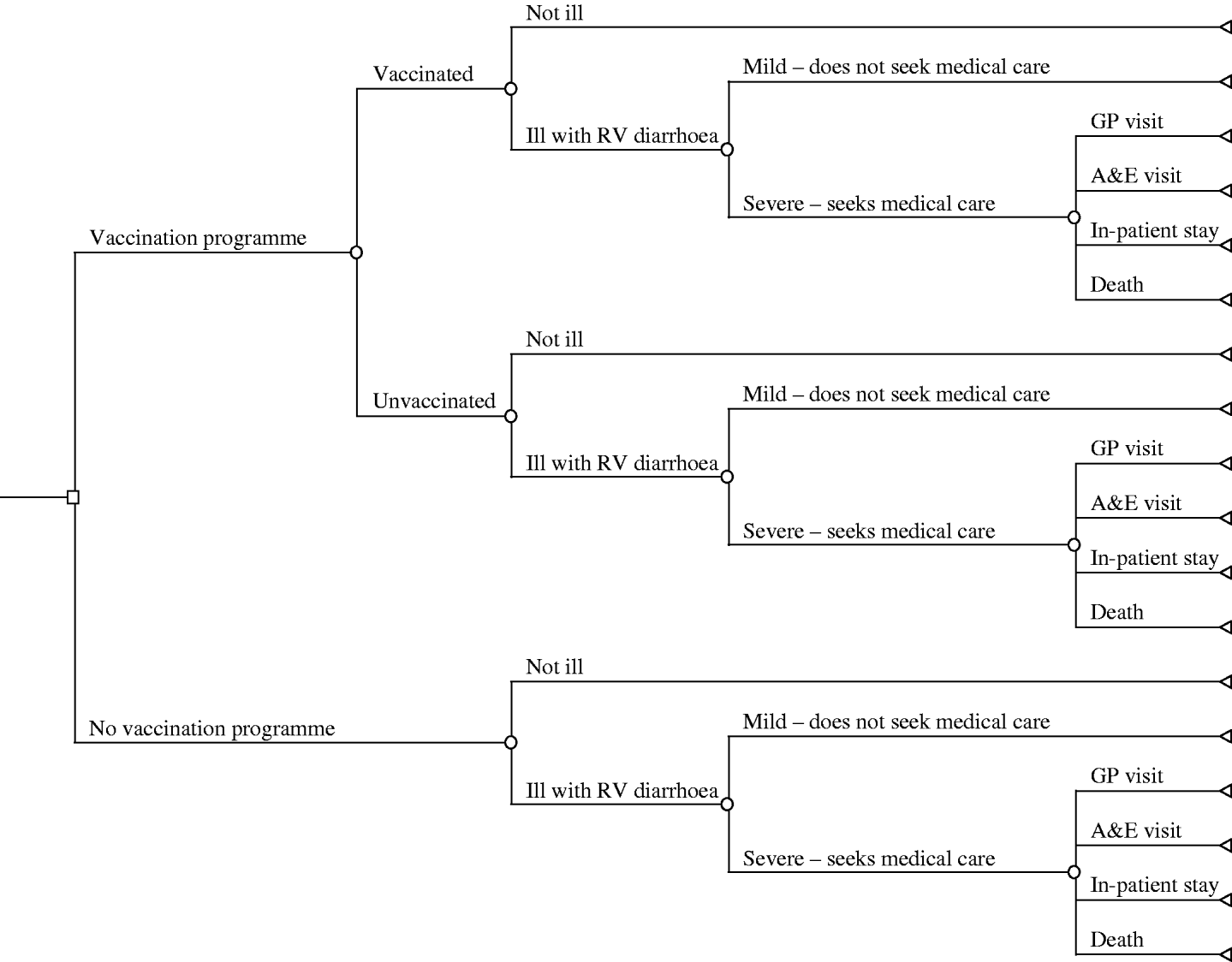
Fig. 1. Decision tree for a rotavirus immunization programme in the United Kingdom.
Currently, with no vaccine programme (the lower branches of the tree) a child is at risk of infection from rotavirus, given by the branch ‘ill with RV diarrhoea’. The subsequent illness may be severe enough to seek medical care or only take a mild form, such that they are cared for at home. If the illness is severe then there are various outcomes and consequences as indicated by the final branches of the tree; the contact may vary from presenting at a GP surgery, to an A&E attendance, or result in hospitalization, while at its most severe rotavirus infection may cause death. A vaccination programme (the upper branches of the tree) results in similar pathways and outcomes, but is augmented by the assumed vaccination coverage rates and the vaccine(s) efficacy.
Study design
The cost-effectiveness analysis was performed from two perspectives: the health sector perspective which includes only the costs incurred by the NHS (the cost of vaccination and the direct costs associated with medical care); and the societal perspective which includes the costs incurred by the health sector as well as the costs incurred by parents (including over-the-counter medicine purchases) and lost productivity. The analysis follows a single infant cohort over a period of 5 years, a period during which the consequences of the illness are most severe; furthermore, it is assumed that the vaccine would be protective for this time. The nature of the virus and composition of the vaccine means that herd immunity is unlikely, as the vaccine only prevents the onset of the disease, and does not provide a barrier for infection, such that children can be vaccinated but still be infectious; furthermore, wild rotavirus strains are likely to remain endemic. All costs are in 2005/2006 pounds sterling. Costs and values published before 2005/2006 were inflated using the appropriate inflation indicator(s) [Reference Netten and Curtis11].
Outcomes are defined in natural units and not quality-adjusted life years (QALYs) as information on utility gains or improvements in quality-of-life due to rotavirus vaccination are not available. As such a cost-effectiveness analysis is undertaken, rather than a cost utility analysis. Initially the difference in cost with and without a vaccination programme is estimated, such that the cost per eligible child is estimated. Subsequent analyses estimate incremental cost effectiveness ratios (ICERs), whereby additional costs are compared to additional outcomes, giving a cost per event (episode, GP visit and hospitalization) avoided and cost per life year gained.
Probability estimates
The probabilities of events and outcomes in the decision tree were derived from published studies and national sources. Table 1 provides a summary of these, and includes the base case estimates used for initial analysis and the best- and worst-case estimates used in the sensitivity analysis.
Table 1. Probability estimates of cumulative incidence of infection, morbidity and mortality by age 5 years, vaccine coverage and efficacy (%)
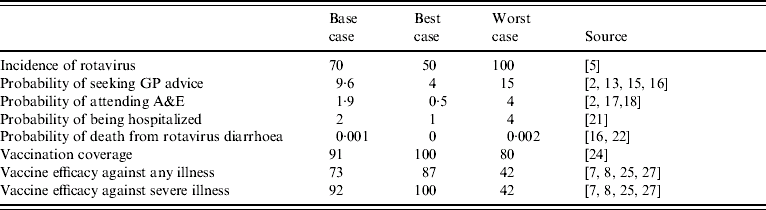
Estimates of the incidence of rotavirus are generally derived from routine voluntary reporting by clinical microbiology laboratories. These estimates, however, can be problematic as they often only reflect the severe cases that are hospitalized. Furthermore, the detection methods used to diagnose enteric viral infections vary widely in terms of their sensitivities. As a consequence of this much of the reported disease incidence is inaccurate. A previous modelling study used an accumulative incidence rate of 70% [Reference Tucker5], implying that from birth to the age of 5 years, each child has a 70% chance of infection. This is used as the base case value in our analysis, and the sensitivity of it is tested using a low value of 50% and a high value of 100%. The upper value, worst-case scenario, implies that by the age of 5 years every child will have had an episode of rotavirus gastroenteritis, while the low value implies that the disease can be asymptomatic.
As stated above rotavirus gastroenteritis can be mild or severe. Of those cases which are severe, treatment will vary according to severity and as such a parent may seek health-care advice by consulting a GP, presenting at A&E, or will result in the admission of their child to hospital. In the most severe instance a child may die. These events are not assumed to be mutually exclusive, such that a child who is hospitalized could have also presented to a GP or at A&E. Note that while vaccination occurs immediately, that is before the age of 12 months, the events that it avoids do not occur until some time in the future. The average age of children with gastroenteritis who presented in the community was 2 years [Reference Lorgelly2], as such it is assumed, for simplicity of the modelling, that all events occur at this age. Therefore it is necessary to discount these costs (and any life years gained); we employ a discount rate of 3·5% per annum, as suggested for use with public sector projects [12], and assume rates of zero and 6% in sensitivity analysis.
It is expected that the majority of severe gastroenteritis will present in primary care. One study has found that 20% of children aged <5 years who attended a GP surgery over a 12-month period had infectious intestinal disease [13]. While the preceding structured surveillance of community-acquired rotavirus found that 48% of those presenting to a GP with acute gastroenteritis had rotavirus [Reference Iturriza Gomara1, Reference Lorgelly2]. If all those who presented with acute gastroenteritis were deemed to have infectious intestinal disease, then this suggests that of a birth cohort of 632 000 children [14], 60 672 would present to a GP with rotavirus gastroenteritis, implying an accumulative risk by the age of 5 years of 9·6% (that is 1/10·4 children would present at a GP with rotavirus gastroenteritis by age 5 years). The preceding cost-of-illness study also found that 20% of parents whose children had symptoms of gastroenteritis telephoned NHS Direct [Reference Lorgelly2], therefore we have included this additional resource use in the cost analysis. An earlier study which also undertook surveillance in the community [Reference Isaacs, Day and Crook15] reported that only 20% of those presenting with gastroenteritis had rotavirus, such that the lower value for sensitivity analysis is 4%. The upper value is derived from a recent paper which estimated the burden of illness in Europe [Reference Soriano-Gabarrò16]; it reported that there could be some 102 293 physician visits (including outpatient visits) annually in the United Kingdom for rotavirus. Notably such a high probability could occur if a severe endemic strain of the disease was to circulate.
Some of those children who visited a GP will be referred to A&E, while parents of other children whose condition is considered severe out-of-hours may self-refer their children. It has been estimated that each year some 250 children (aged <5 years) per 1000 attend an emergency department, that is a quarter of all children aged <5 years [17]. Diarrhoea is one of the top five medical presentations at A&E for children [Reference Armon18] and further analysis has shown that of the 1198 children aged <5 years who attended A&E during a 4-month period in 1999, 192 had diarrhoea, that is 16% of attendees [Reference Armon18]. Assuming a similar incidence of rotavirus as found in the community [Reference Iturriza Gomara1, Reference Lorgelly2] would imply a cumulative risk of 1·9%, that is 1/52 children would present at A&E with rotavirus gastroenteritis by the age of years. Similar rates have been found in Europe; in the Basque region the incidence rate of rotavirus presenting at emergency departments was 2·2/100 children aged <4 years [Reference Cilla19], while the incidence of emergency attendance for rotavirus gastroenteritis in a placebo group in a vaccine trial [Reference Joensuu20] was 2·6/100 infants. The lower value used in our sensitivity analysis implies that rather than attend A&E, the majority of parents consult with an out-of-hours GP or use NHS Walk-in Centres, while the high value assumes a severe endemic strain.
In its most aggressive form children with rotavirus gastroenteritis can be extremely dehydrated and require hospitalization. A study of laboratory reports found that 54% of admissions for intestinal infection disease had rotavirus, while 34% of non-infectious gastroenteritis hospitalizations also had rotavirus [Reference Ryan21]. Given the number of hospitalizations over a period, this suggested that 5·2/1000 children aged <5 years are hospitalized with rotavirus annually, which gives a cumulative incidence by the age of 5 years of 1/38. This incidence is expected to be upwardly biased as the investigation was undertaken within a hospital setting; therefore, we have used a slightly lower incidence of 2%, that is 1/50 children will be hospitalized with rotavirus gastroenteritis by the age of 5 years. The best-case probability estimate assumes limited in-patient care is required, while the worst-case estimate of 4% reflects what might happen during a severe outbreak.
In their study Parashar et al. [Reference Parashar3] estimate that in industrialized countries death can occur in 1% of cases, however, in the United Kingdom death from rotavirus is rare. Crowley et al. [Reference Crowley, Ryan and Wall22] combining public health laboratory reports and mortality data on gastroenteritis estimated that seven deaths per year of those aged <5 years could be attributed to rotavirus gastroenteritis; that is 2·3 deaths per million children annually. Therefore, the base case probability is 0·00001 (or 0·001%) which reflects the cumulative incidence by age 5 years. Soriano-Gabarró et al. [Reference Soriano-Gabarrò16] using Parashar et al.'s [Reference Parashar3] calculations estimate that there were some 14 deaths annually in the United Kingdom; this provides a worst-case estimate and further implies that many deaths can not be prevented because the vaccine only offers protection for those children aged ⩾6 months. The best-case estimate assumes no deaths from rotavirus gastroenteritis.
To determine the effect that vaccination will have on these outcomes, we need to estimate the likely uptake of the vaccine and its efficacy. Currently in the United Kingdom there exists some scepticism about the safety of childhood vaccinations. This may be due to ‘media hype’ and an unsubstantiated link between the measles, mumps and rubella (MMR) vaccine and autism [Reference Offit and Coffin23]. This appears to have had some effect on immunization coverage rates. In 2005–2006 only 84% of children by age 2 years had been immunized against MMR with the combined MMR vaccine, higher than 81% in the previous year, but much lower than the peak of 92% in 1995–1996 [24]. Other vaccination rates [for diphtheria, tetanus and polio (DTP), pertussis and Haemophilus influenzae b (Hib) and meningitis C], however, remained similar across time. To their merit, both vaccines that have been recently introduced are oral, and as such parents are likely to regard them as safer than those that are administered by subcutaneous or intramuscular injection. As both vaccine courses are expected to be complete before 12 months of age, and have similar schedules to the DTP vaccine, the coverage rate used in the model is that for DTP at year of first birthday, 0·91 [24]. The worst-case estimate is the current coverage rate for the MMR vaccine, and the best-case estimate assumes blanket coverage, such that every child is vaccinated against rotavirus gastroenteritis.
As two vaccines have received licensure in a number of countries, the model attempts to remain impartial and as such the efficacy rates used reflect mean efficacy. The Rotarix vaccine has performed well in clinical trials. The initial pilot in Finland with 405 children reported an efficacy of 72% over a 2-year period against any diarrhoea, and an efficacy of 85% against severe diarrhoea [Reference Ruiz-Palacios7, Reference Vesikari25]. Other trials in Latin America and Singapore also show that the vaccine is well-tolerated, and after two doses, 61–91% of vaccinated infants developed rotavirus-specific IgA antibodies [Reference De Vos26]. The Rotateq vaccine has also been shown to be efficacious and safe; clinical papers report that it has an efficacy of 74% against any rotavirus gastroenteritis and is up to 98% efficacious against severe rotavirus gastroenteritis [Reference Vesikari, Giaquinto and Huppertz6, Reference Clark27]. It is important to point out, however, that a different scoring system was used across the respective trials and as such direct comparisons of efficacy against ‘severe’ disease should not really be made [Reference Vesikari, Giaquinto and Huppertz6]. This aside, an efficacy of 73% was employed as the base case for any diarrhoea, and 92% for severe diarrhoea, where as described above severe disease is categorized as seeking health care from a health professional. The best- and worst-case values reflect the highest and lowest values from reported confidence intervals around each efficacy. Notably a recent systematic review of rotavirus vaccines (Soares-Weiser et al. [Reference Soares-Weiser28]) found, from pooled analysis, that rhesus and bovine vaccines had an efficacy against one episode of rotavirus diarrhoea of 41%, while human vaccines had an efficacy of 58%. However, heterogeneity was a problem when comparing studies, and the review did not include the latest generation of vaccines, and as such is not used to inform this analysis.
Cost estimates
Table 2 presents the cost estimates used in the model. These are from routine sources and where necessary have been inflated to 2005/2006 prices [Reference Netten and Curtis11]. Specifically, the cost of a telephone advice from NHS Direct, the unit cost of an ICU attendance and the cost of prescribed medicines have been inflated using the Hospital and Community Health Services (HCHS) pay and price index; while the cost of over-the-counter medications has been inflated using the retail price index.
Table 2. Rotavirus gastroenteritis cost estimates (£ sterling, 2003/2004)
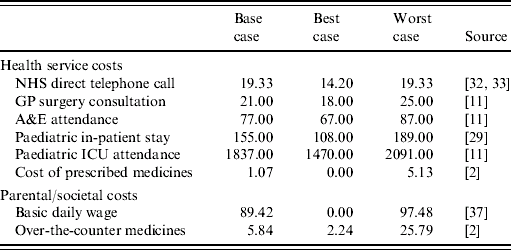
Little is known about the cost of a child dying whilst in hospital. We have assumed that due to the severity of the illness the child will be in a paediatric intensive care unit, the average cost of which is £1837 per bed day [Reference Netten and Curtis29]. The best and worst cost estimates reflect the lower and upper interquartile range estimates. For a child who is hospitalized our estimate of cost involves multiplying the unit cost of bed day in a paediatric ward [Reference Netten and Curtis11] by the average length of stay. Estimates of the average length of stay for a rotavirus sufferer range from 5·5 days [Reference Noel30] to 2·2 days [Reference Carlin9]. We have chosen a base case of 3·8 days, similar to the average and also that reported recently by Gil et al. [Reference Gil31]; the best and worst estimates are those of Carlin et al. [Reference Carlin9] and Noel et al. [Reference Noel30], respectively. Attendance at A&E is estimated to cost £77 per visit (lower cost investigations are assumed), with best and worst cost estimates of £67 and £89, respectively [Reference Netten and Curtis11]. The cost of attending a GP surgery is also estimated using routine cost data, but it is augmented by the additional cost of prescribed medicines; such that the cost to the health service of consulting a GP is the unit cost of a visit plus the cost of prescribed medicines. Lorgelly et al. [Reference Lorgelly2] found that the average cost of prescriptions for rotavirus gastroenteritis patients who consulted a GP was 95 pence in 2002. This value has been inflated to 2005/2006 prices, while the best and worst cost estimates reflect the minimum and maximum prescription costs as reported by Lorgelly et al. Lastly, the cost of a telephone call to NHS Direct was estimated to be £15.11 in 1999/2000, again this has been inflated [32]. The worst-case estimate is the same as the base case, while the best-case value assumes economies of scale in the long run [Reference Munro33].
To date neither Rotarix nor Rotateq have been licensed for use within the United Kingdom, such that no information exists as to what they will cost the NHS. The recently introduced 5-in-1 vaccine, Pediacel® (Sanofi Pasteur MSD Ltd, Maidenhead, UK), has a net price of £19.94 per syringe [34]. This is a combined vaccine for diphtheria, tetanus, whooping cough, Haemophilus influenzae type b meningitis and polio, and is given in three doses from age 2 months onwards. Rotateq, although administered orally, is also given in three doses and as such we have assumed a similar price of £20 per dose, or £60 per completed course. Rotarix, also oral, requires just two doses, however, given competitive forces one can expect them to be priced similarly, so a cost of £60 per completed course is also assumed, that is a price of £30 per dose. Note that much of the subsequent modelling is based on a three dose vaccine, but the results are also applicable to a two-dose vaccine. Further, it is assumed that either vaccine will fit comfortably into the current national immunization schedule and as such rotavirus vaccination will not require additional GP visits. Notably, as the vaccines are oral, they will require refrigeration; the model assumes the cost of this vaccine administration, and any possible target (incentive) payments made to GPs, are subsumed in the cost of the vaccine as included in the model. This cost will be subject to specific sensitivity analysis.
The human capital approach was used to estimate the productivity costs of rotavirus gastroenteritis. We assumed that production loss arises from parental absence from work and premature death. An important consideration when estimating parental lost productivity is the complexity of the family unit in terms of whether families are supported by one or two incomes. The young age of the children involved, often means that many mothers may not work or only work part-time. In this analysis it is assumed that only full-time employment of one parent in lone-parent households or of both parents in two-parent households would lead to a loss of earnings when caring for a sick child. As women generally earn less than men, we assume that the mother would incur this wage loss in a double-income family.
Given, 73% of families with children have parents who are married or cohabiting (that is 27% of families with dependent children are headed by a lone parent), and in 56% of these families both parents work, and 39% of mothers are employed full time; while in lone-parent families, 49% of lone parents (be it the mother or father) work full time, we have estimated that 33% of mothers/lone parents would have a forced absence from work [Reference Walling35, 36]. This is similar to what was found in the cost-of-illness study [Reference Lorgelly2], where 40% of parents took time off paid work.
For a child that is hospitalized the model assumes that a parent foregoes earnings for the period that the child is in hospital (3·8 days, range 2·2–5·5) and also for an additional 3 days, during which time the child fully recovers. The value of foregone earnings was derived from the median weekly wage, which was £447 [37]; while the lifetime lost productivity of a deceased child is estimated using a median annual salary of £23 600. Many day-care centres now require that a child is symptom free for a period of days prior to their return, therefore, we have assumed that, in the absence of other forms of child care, a parent will have to forego earnings, stay at home and care for their child for a period of 3 days in all illness outcomes, including when the child has only mild disease. Note for sensitivity analysis these days off take a best-case estimate of zero days and a worst-case estimate of 5 days.
For a child who dies we have assumed that the parent(s) may forego earnings in terms of taking time off work to grieve for their loss. As discussed above some parents do not work and, therefore, will not forego earnings, but in the model we have implicitly valued all time away from usual activities, be it paid work, unpaid work, child care or leisure, as a loss and as such it is valued as if it were paid employment. Furthermore, parents will also suffer other ‘psychic costs’ from the death of their child, but these can not be valued, and as such are not included in the cost estimates of the model.
Finally, for those parents who consult a GP or do not seek the advice of a health professional, the model assumes that they incur the expense of over-the-counter medication purchases. The cost-of-illness study [Reference Lorgelly2], found that many parents purchased remedies, such as rehydration fluids, over the counter. This study estimated that the average cost of such expense in 2002 was £5.21, ranging from nothing to £23. The values in Table 2 for over-the-counter medicines reflect the adjusted 2005/2006 cost.
Sensitivity analysis
Sensitivity analysis was conducted on all the variables in the model that are considered to be uncertain. From the base-case estimates univariate sensitivity analyses using the best- and worst-case estimates in Tables 1 and 2 were performed. Threshold analysis was also undertaken to establish the break-even price of the vaccine.
RESULTS
From a societal perspective a vaccination programme for rotavirus gastroenteritis is found to dominate the alternative of having no programme. The total cost per child in the population is estimated to be £79.19 under a scenario of vaccination, while under the current scenario, with no vaccination programme, the total cost per child is £86.33. Therefore, for an annual birth cohort of 632 000 children, rotavirus vaccination could provide net savings to society of £4.5 million, under ‘base case’ assumptions.
However, from the health service perspective, a vaccination programme is estimated to be more costly. Table 3 presents the results of these additional costs. The introduction of a rotavirus immunization programme (using our base case estimates) would cost the NHS £42.49 for every eligible child. Using the birth cohort, this would result in a net health sector cost of £26.7 million over a 5-year period. This cost increases if the outcome is more narrowly defined, that is the ICER for preventing an episode of gastroenteritis is estimated to be £60.41, while the cost per life year saved is £177 212, well above any recognized acceptable cost-effectiveness threshold.
Table 3. Incremental cost effectiveness ratios (ICER), health sector perspective (£ sterling, 2005/2006)

Univariate sensitivity analyses found these base case estimates of cost effectiveness to be relatively sensitive to some of the best- and worst-case scenarios. Tables 4 and 5 present the results for the five most sensitive probability and cost parameters in the model (excluding the cost of the vaccine). From the health sector perspective (Table 4) varying the probability that rotavirus gastroenteritis, from a best-case estimate of 1% to a worst-case estimate where 4% of the cohort are hospitalized over a 5-year period, results in the net cost per eligible child varying between £46.89 and £33.08, respectively. Note, that the worst-case probability estimates give lower ICERs because the ‘no vaccination’ arm is more sensitive and has greater variation than the ‘vaccination programme’ arm, such that the magnitude of the cost increases are greater without a vaccination programme relative to having a vaccination programme. Notably, none of the best or worst estimates significantly change the result; the cost to the health service is always more with a vaccination programme, than without such a programme.
Table 4. Sensitivity analysis, net cost per eligible child, health sector perspective (£ sterling, 2005/2006)
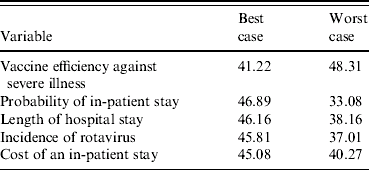
Table 5 shows that from the societal perspective, while dominance is maintained in most instances (indicated by the negative values reflecting net savings), when parents take no time off work or lose no income while away from employment, a vaccination programme may be more costly than no programme. If parents had no time away from work then a vaccination programme would cost society £30.81 for every eligible child, and if parents took time off employment but did not forego earnings, then a vaccination programme would cost society £32.58 for every eligible child. Under these scenarios vaccinating a birth cohort would result in net societal costs of between £19.4 and £20.6 million.
Table 5. Sensitivity analysis, net cost per eligible child, societal perspective (£ sterling, 2005/2006)
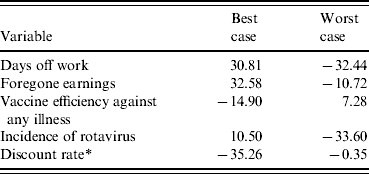
A negative value indicates that the vaccination programme dominates no vaccination programme.
* For the discount rate, the best-case value equates to zero and the worst-case value equates to 6%.
The results of threshold analysis, to establish the break price of the vaccine, are presented in Figure 2. This graph shows that from the societal perspective, using our base case estimates, net savings per eligible child are possible up to a price of £22.61; that is, the total vaccination course could cost up to £67.83 before society would negate any gains from the introduction of a vaccination programme. However, on the other hand, a dose of the vaccine would have to cost less than £4.51, or less than £13.53 per course, for the health sector to ever realize any cost savings.
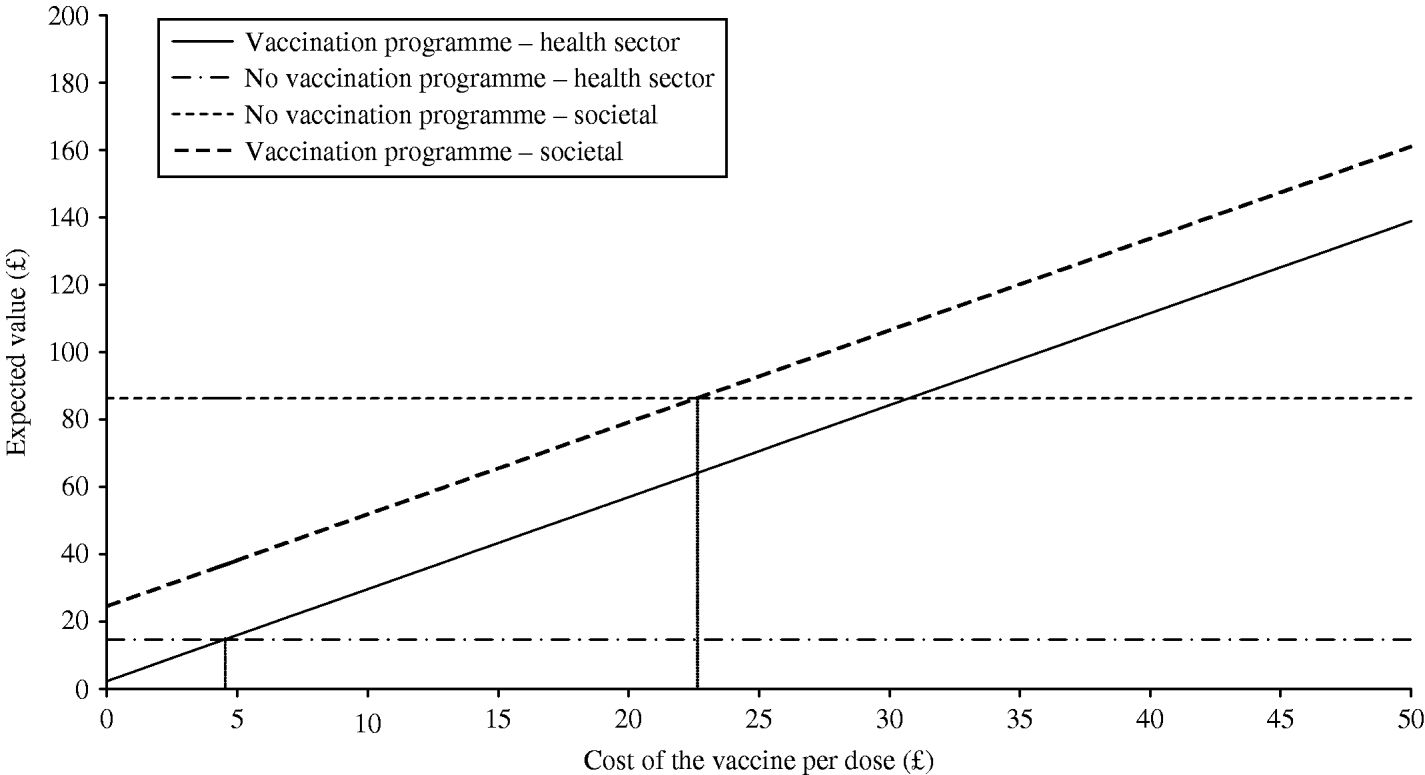
Fig. 2. Threshold analysis, cost of the vaccine and expected value with and without a vaccination programme.
DISCUSSION
Our analysis, using the latest estimates of disease burden and vaccine efficacy, suggests that a rotavirus vaccination programme in the United Kingdom, where a course of the vaccine costs £60, would not be cost effective. Such a programme would result in net costs to the health service of £26.7 million. This net cost, however, may be considered worthwhile (that is the health service may be willing to pay £42.29 per child) if there is a sufficient improvement in the quality-of-life of the children and parents affected by rotavirus gastroenteritis. In our review of the evidence we failed to find any studies that had measured quality-of-life or utility gains as a result of rotavirus immunization. This, however, is not surprising given a consensus on how to best measure children's quality-of-life has yet to be reached [Reference Harding38–Reference Raat40].
From the societal perspective, however, we do find that cost savings are possible with the introduction of a vaccination programme. These savings are substantial, although could be negated if parents do not take time off work or do not lose income while taking time off to care for their children. However, in today's society where many mothers return to employment after childbirth, one would expect that they or their partner or a family member would have to take time off employment (or have time away from their usual activities) to care for their ill child. It is also the norm that such time away from work would be covered by parental leave, such that income is not lost, however, taking a global societal perspective such time off would still be a loss of productivity and society would still bear this cost. Therefore, despite these sensitivities, one can argue that rotavirus vaccination is cost effective from the societal viewpoint, although lower incidence rates and a less efficacious vaccine has the potential to negate these cost savings.
To date there is only one other published cost-effectiveness analysis which evaluates the new generation of rotavirus vaccines [Reference Isakbaeva10]. This has been undertaken within Uzbekistan, a low-income country which is eligible for Global Alliance for Vaccines an Immunization (GAVI) funding, such that they may be an early adopter of a rotavirus vaccine. Isakbaeva et al. [Reference Isakbaeva10] find, taking a health sector perspective, that while a vaccination programme would not be strictly cost saving, it would be considered ‘very cost effective’ given the WHO's cost per disability adjusted life year (DALY) saved threshold, that is an ICER less than per capita GDP [41]. Sensitivity analysis shows that their results are greatly influenced by the mortality rate, which is unsurprising given they are a low-income country (although with a developed health-care system) where it is estimated that 17% of all deaths from diarrhoea can be attributed to rotavirus [Reference Parashar3]. Isakbaeva et al. also find, as in our analysis, that the evaluation is sensitive to the vaccine's efficacy, the rate of hospitalizations and the price of the vaccine. It would appear, therefore, that these parameters will be important when determining the (un)certainty of decisions on introducing a routine vaccination programme.
Given that the current evaluation finds that the vaccine is not cost effective (that is cost saving) from the health sector perspective but is from the societal perspective, it raises the issue as to which perspective should be given priority when informing policy. While the Joint Committee on Vaccination and Immunisation (JCVI) is currently not explicitly obliged to consider cost effectiveness when deciding whether to approve a new vaccine for introduction into the vaccination schedule; the health technology equivalent, the National Institute for Health and Clinical Excellence (NICE), does require evidence of cost effectiveness. Furthermore NICE guidance [42] states that ‘[t]he perspective adopted on costs should be that of the NHS and PSS (Personal Social Services). If the inclusion of a wider set of costs or outcomes is expected to influence the results significantly, such analyses should be presented in addition to the reference case analysis’ (p. 22). They argue that this is consistent with their objective of maximizing health gain from available resources. If the JCVI also takes a similar view this would suggest that at a cost of £20 per dose, for a three-dose vaccine, rotavirus vaccination would not be regarded as efficient. Only if the price were set to around £13 per completed course, would it be considered value for money.
However, it is important to consider the societal perspective, as this provides information on the equity implications of introducing an immunization programme. Differing cost effectiveness across perspectives reflects the fact the rotavirus gastroenteritis is a significant burden on parents and families. The cost-of-illness study [Reference Lorgelly2] found that approximately 7% (£0.8 million) of the total societal burden of rotavirus gastroenteritis was incurred by parents and families. If a decision was made to introduce the vaccine then this would shift some of the burden of illness from parents to the health service.
As indicated above, there is a need for further research in this area to estimate outcome in terms of quality-of-life and/or QALYs (a recent paper by Griebsch et al. [Reference Griebsch, Coast and Brown43] provides a good discussion of the use of QALYs in paediatric care; see also a recent paper by Rheingans et al. [Reference Rheingans, Heylen and Giaquinto44] which discusses the use of QALYs as an outcome measure in the evaluation of vaccines). This would allow for a cost-utility analysis to be undertaken, which could better inform a policy decision, in terms of making comparisons to cost-effectiveness thresholds (as Isakbaeva et al. [Reference Isakbaeva10] have done with DALYs, a common outcome measure in low-income and developing countries). An alternative would be a cost–benefit analysis, whereby the outcome is in monetary units, such that costs can be directly compared to benefits, and the net benefits (or costs) of a vaccination programme could be estimated. One way to do this would be to elicit parents' willingness to pay (WTP) to have their child vaccinated against rotavirus gastroenteritis. Following the withdrawal of the earlier rotavirus vaccine, Rotashield, research was undertaken to determine what risk (in terms of intussusception) parents would be willing to accept to obtain the other benefits of the vaccine [Reference Sansom45]. The authors found that parents were willing to pay more (median WTP US$110) for a risk-free vaccine than one with a risk of 1400 cases of intussusception a year in the United States (median WTP US$34). Such an elicitation exercise could be informative to our study as it would allow some reflection of the value that parents place on inconvenience and other intangible losses. Such costs may be more substantial in an evaluation of rotavirus vaccination as, unlike most childhood illnesses for which vaccines exist, gastroenteritis does not result in long-term chronic health and is generally not fatal.
Finally, our analysis was based on the short run, in that it only considered the immediate costs and consequences of an episode of illness and infection. The nature of gastroenteritis rotavirus is such that over time with greater exposure to the infection, a child will develop natural immunity, thus, the first or second episodes of illness are more severe than subsequent infections which are often asymptomatic. The vaccination, therefore, acts as an alternative to building this natural resistance. It is unknown how long this natural resistance lasts, but similarly the length of protection from vaccination is also unknown. Infection in the elderly is not uncommon [Reference Marshall46, Reference Anderson and Weber47], so there is some possibility that childhood vaccination now could provide protection in generations to come. If this is not the case, there may be some argument to undertake vaccination of the elderly, as well as children, as is the case with influenza vaccination. This, however, would require further research to establish its efficacy, effectiveness and cost effectiveness in this population.
DECLARATION OF INTEREST
None.









