Introduction
Tick-borne rickettsioses (TBR) include nearly 20 diseases caused by different species of spotted fever group rickettsiae, placing them among the most significant zoonoses worldwide [Reference Fang, Blanton and Walker1]. Of these, in the Americas, Rickettsia rickettsii causes the most severe cases of TBR and has a high mortality rate in untreated patients [Reference Fang, Blanton and Walker1–Reference de Oliveira3]. Human infections with R. rickettsii have been reported throughout the Americas; it is known as Rocky Mountain spotted fever (RMSF) in North America, but also has regional names like ‘fiebre maculosa Brasileira’ (Brazil) or ‘fiebre de Tobia’ (Colombia) [Reference Oteo4]. The ecology of its transmission varies according to the region: in North America, the most common natural vectors are Dermacentor ticks and peri-urban Rhipicephalus sanguineus s.l., in Central and South America Amblyomma ticks, and R. sanguineus s.l. is potentially a vector in Costa Rica and Brazil [Reference Oteo4–Reference Levin6]. In addition, R. rickettsii has been reported in the ticks Haemaphysalis leporispalutris, Amblyomma tenellum (previously called Amblyomma imitator), Dermacentor nitens, Amblyomma americanum and Amblyomma parvum [Reference Labruna7].
Panamanian cases of RMSF were first reported in 1950, with two fatal cases among five patients [Reference de Rodaniche and Rodaniche8, Reference Calero, Nunez and Silva Goytia9]. From 2004 to 2013 seven more cases were confirmed, with six of them were fatal [Reference Estripeaut10–Reference Bermúdez13]. In addition, more evidence of the circulation of TBR in Panama was obtained in surveillance studies [Reference Silva-Goytia and CALERO14, Reference Bermudez15]. Except for one case from a peri-urban area in Panama City [Reference Calero, Nunez and Silva Goytia9] and another from a heavily forested area [Reference De Lucas12], the majority of patients came from rural agricultural regions. In Panama, R. rickettsii was isolated from A. mixtum (formerly called Amblyomma cajenenense) [Reference De Rodaniche16] and R. sanguineus s.l. has been considered a putative vector [Reference Calero, Nunez and Silva Goytia9]. Since these species differ in their environmental and host preferences [Reference Bermúdez13], it is important to understand their ecology in Panama in order to comprehend and prevent transmission of R. rickettsii. This paper reports the clinical and pathological description of two new cases of R. rickettsii spotted fever and presents a new urban scenario for this disease in Panama.
Case 1
The first case occurred on 28 February 2017. An 8-year-old male patient from El Valle, a rural community in Coclé Province, was admitted to Hospital Aquilino Tejera (HAT) in Coclé Province; he presented with a 5-day history of intense headaches, fever, diarrhoea, vomiting and abdominal pain. On the third day after onset of fever, a generalised skin rash appeared. Laboratory tests at the time showed a platelet count of 40 000/mm3, leucocytes at 5000/mm3 (93% neutrophils) and haemoglobin measured at 10.3 g/dl. The initial suspicion was dengue fever. The patient was treated at a primary health centre with antipyretics and amoxicillin, and later with ampicillin, clindamycin and gentamicin at the HAT. One day after his admission to the HAT, he was transferred to the Hospital de Especialidades Pediátricas Omar Torrijos (HEP) in Panama City in critical condition. Laboratory tests then indicated thrombocytopenia (with a platelet count of 22 000/mm3), leucocytes at 5800/mm3 (88% neutrophils), and low haemoglobin (9.5 g/dl) and haematocrit 27.8%. Bilateral alveolar infiltrate was observed on the radiograph. The patient's health deteriorated until he developed respiratory insufficiency and heart failure. Despite intensive treatment, he died on 2 March 2017. Molecular analyses and culture testing for dengue and other arboviruses were negative.
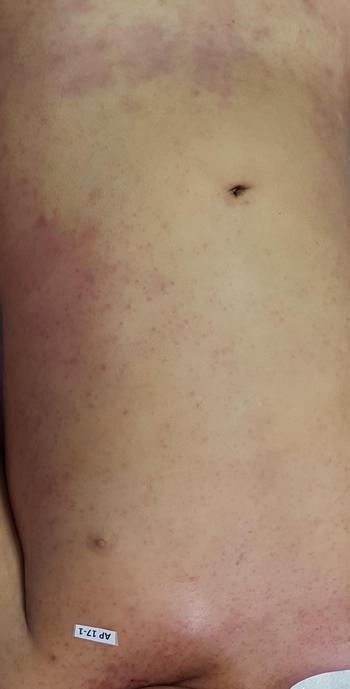
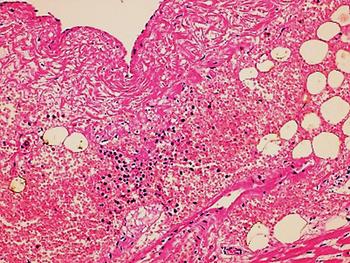
The autopsy revealed petechiae and generalised oedema (Fig. 1), congestion and focal haemorrhage in multiple organs associated with generalised vasculopathy of small-medium vessels with partial fibrin microthrombi formation, pericardial, pleural and peritoneal effusion, and interstitial pneumonitis with diffuse alveolar damage (Fig. 2). Additionally, the patient had foci of endo-myocarditis, acute renal tubular necrosis and chronic hepatitis.
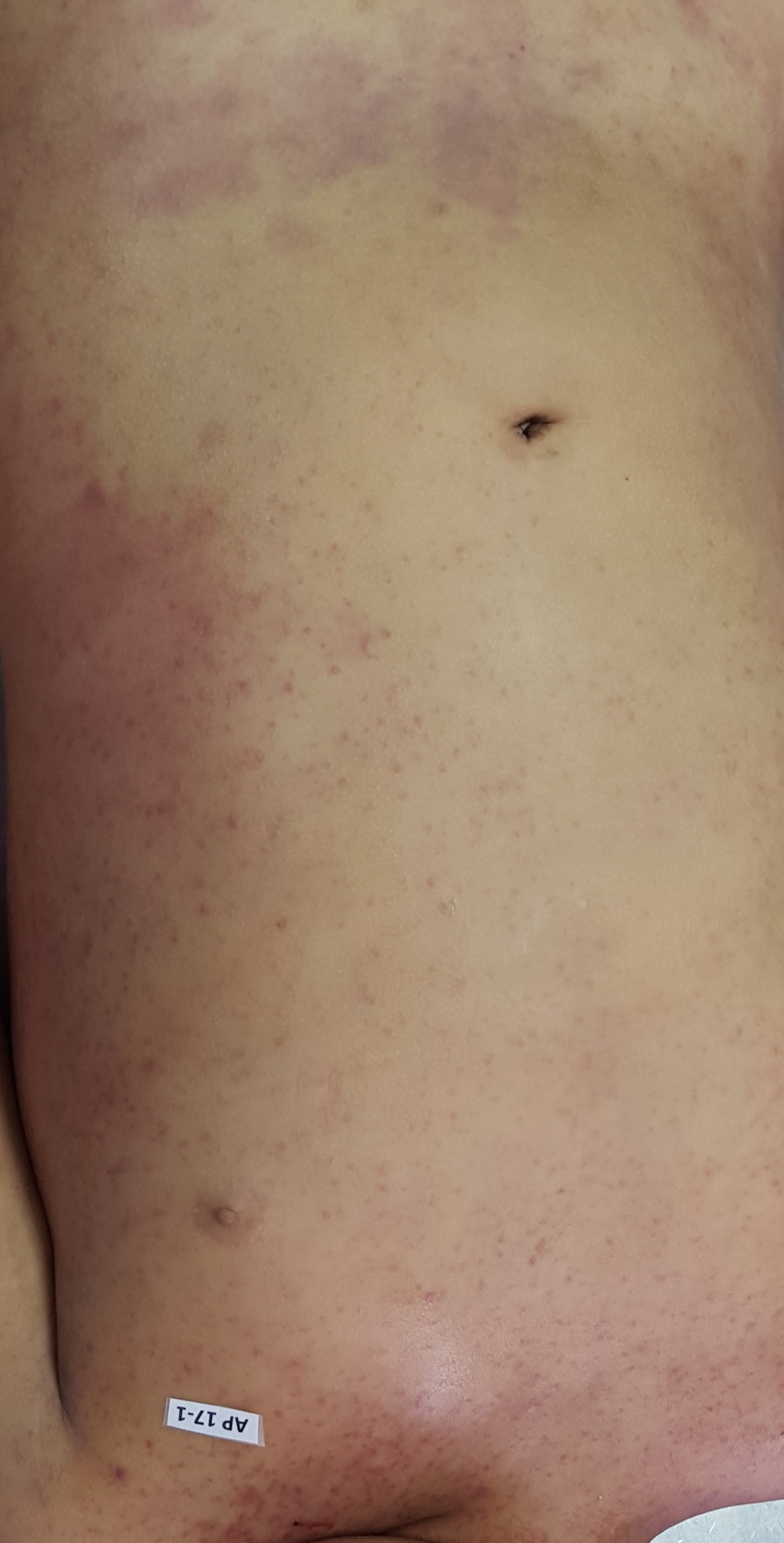
Fig. 1. Petechiae and purpuric lesions on trunk and extremities.
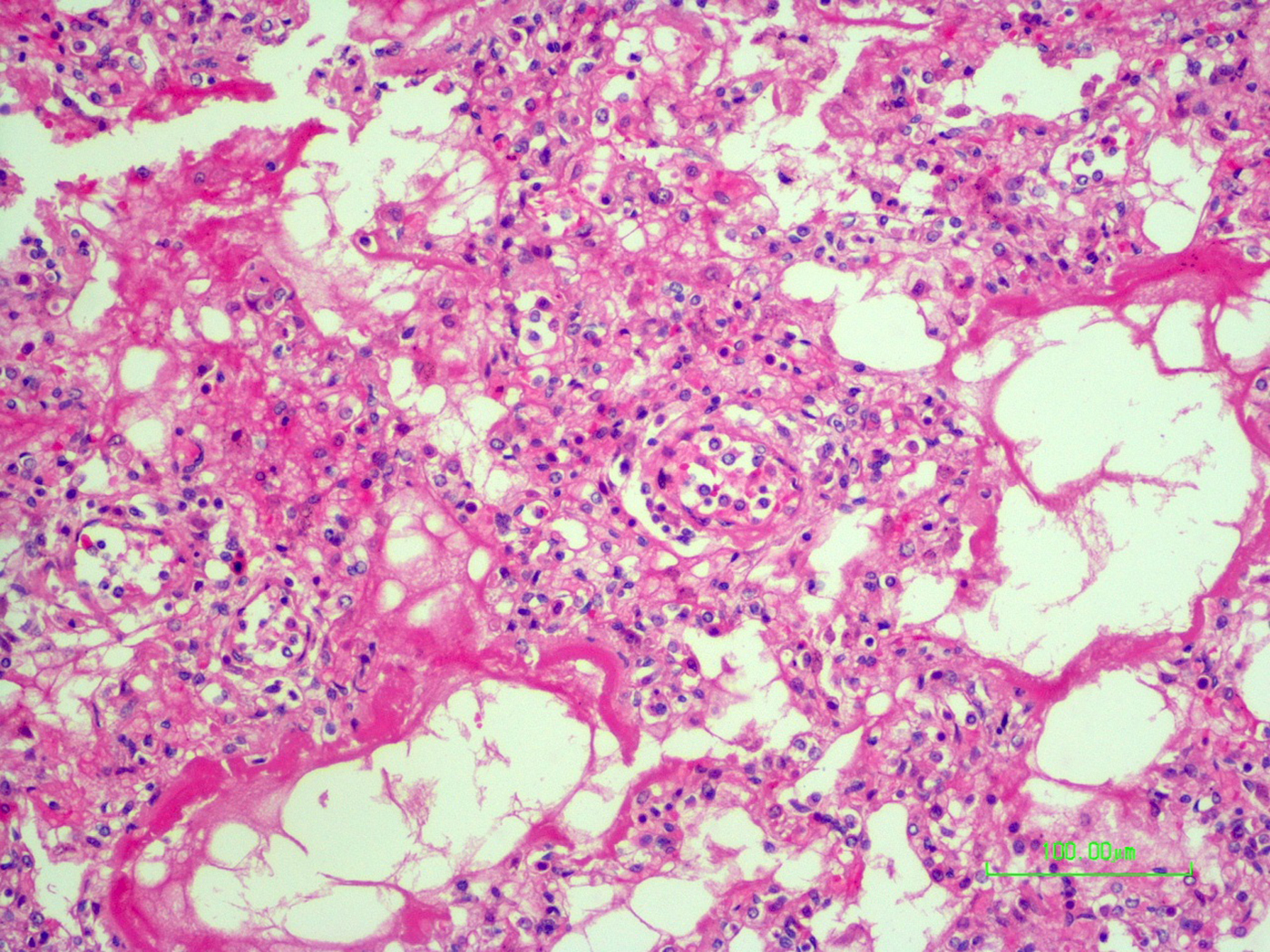
Fig. 2. Interstitial pneumonitis and diffuse alveolar damage (HE 100×).
Multiple samples of the liver were recovered during the autopsy and were used to test for TBR by PCR. DNA was extracted using the DNeasy tissue kit (Qiagen, Hilden, Germany), following the manufacturer's instructions for tissue samples. The sample was positive by PCR for Rickettsia-specific primers Rr190.70 and Rr190.701 [Reference Roux, Fournier and Raoult17]. PCR products were purified using ExoSap (USB), and sequenced with an automated sequencer (Applied Biosystems model ABI Prism 3130 × l Genetic, California, USA). Sequences (GenBank MF678553) had 100% homology with R. rickettsii (GenBank KX363464).
Relatives of the patient did not experience recent tick bites but mentioned travel to the highlands of Chiriquí Province.
The home was a concrete building surrounded by pasture. The house was visited on 4 April 2017, but no ticks were found during the visit in either the house or pasture.
Case 2
The second case occurred on 2 March 2017. A 15-year-old male patient from an urban neighbourhood of Panama City was admitted to the Complejo Hospitalario Armodio Arias Madrid, with an 8-day history of intense headaches, fever, general malaise, myalgia, fatigue, abdominal pain and 2 days of convulsions and shivers. Laboratory tests showed thrombocytopenia (low platelet count of 42 000/mm3), normal leucocytes (6900/mm3), with increased neutrophils (90.4%), abnormal creatinine (5.28 mg/dl), Ca2+ (6.9 mg/dl), inorganic phosphorus (8.0 mg/dl), Mg2+ (3.5 mg/dl), total serum protein (5.5 mg/gl), albumin (2.8 mg/dl), total bilirubin (3.93 mg/dl), direct bilirubin (3.3 mg/dl), lactate dehydrogenase (1077 U/l), aspartate aminotransferase (219 U/l), alanine aminotransferase (87 U/l) and a prothrombin time (14.5 s). Upon admission, serum and cerebrospinal fluid were obtained for culture, molecular and serological tests for dengue, Leptospira and Rickettsia. All tested negative. Despite intense medical efforts, the patient died on 3rd March.
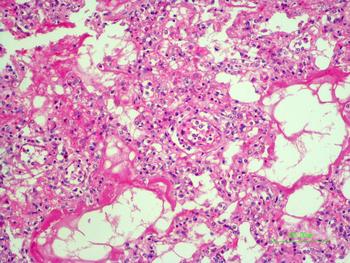
In the second case, the autopsy revealed jaundice, haemorrhages in temporal muscles, brain oedema, and multiple haemorrhages in epicardium; haemorrhagic myocarditis (Fig. 3); pericardial and pleural bilateral effusions; oedema and focal haemorrhages in both lungs; ascites, hepatic lesions characterised by portal triaditis; mild endothelial proliferation and perivascular mononuclear infiltrates in the lobes and splenomegaly. Microscopic examination of haemorrhagic lesions confirmed the presence of vasculitis.
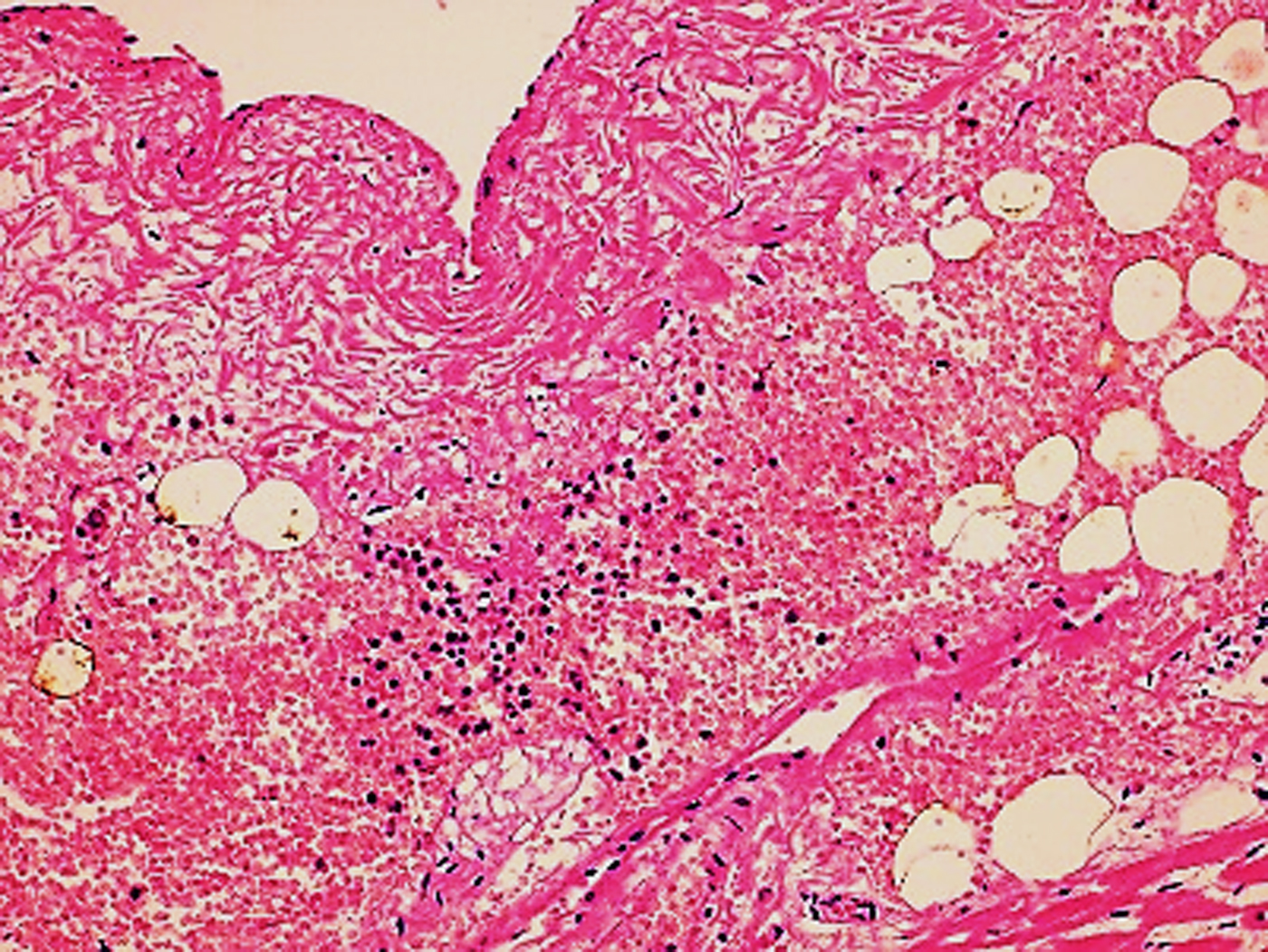
Fig. 3. Micro haemorrhagia in the myocardium (HE 10×).
New liver samples were obtained from the autopsy and tested for hantavirus, leptospirosis and TBR (PCR). Tissues were negative for hantavirus and Leptospira. Using the same methods described above, the sample tested positive by PCR with Rickettsia-specific primers and the sequences (GenBank MF678554) showed a 100% homology with R. rickettsii (GenBank KX363464). One sequenced data were submitted to GenBank under the accession number MF678554.
An ecological approach was used to evaluate the house on 29 March and 4 April. This structure is a concrete building at the end of a street that borders a stream. The parents of the patient reported no travel before the onset of symptoms. Ticks were collected from internal and external walls, and from household dogs. These ticks were identified morphologically as R. sanguineus s.l. and 57 were analysed by PCR, using the same primers and methods described above. From these samples, DNA of R. rickettsii was detected in five adult ticks (8.7%). The sequenced data were submitted to GenBank under the accession numbers MF678548, MF678552. To evaluate the circulation of TBR in others in the household, blood was collected from people (4) and dogs (14) but all samples were negative by immunofluorescence assays (IFA).
Discussion
Compared with other cases of TBR, the course of disease caused by R. rickettsii is rapid and may be lethal if the patient does not receive appropriate antibiotic therapy early in the course of the disease. Thus, timely treatment improves the patient's chances of survival especially, in children, elderly and immunosuppressed persons [Reference Minniear and Buckingham2, Reference Alvarez-Hernandez18, Reference Álvarez-Hernández19]. The clinical presentations of both patients were similar to other recent cases in Panama, with sequential events being a sudden decrease in platelet counts, an increase in liver enzymes, and an abrupt deterioration of health during the fourth or fifth day after the onset of symptoms [Reference de Oliveira3, Reference Estripeaut10, Reference Tribaldos11]. Oral amoxicillin, ampicillin, and clindamycin in both cases were ineffective, confirming the need to use doxycycline as early as possible [Reference de Oliveira3, Reference De Lucas12, Reference Alvarez-Hernandez18, Reference Argüello20]. Both autopsies revealed severe damage to the organs, characterised by oedema and haemorrhage, which are common in cases of RMSF.
Two factors negatively influenced the diagnosis of these cases, which continues to be a significant challenge for public health workers. One factor is that the early clinical presentation of TBR is similar to other infectious diseases with a higher prevalence; the classic petechial rash is not always evident at initial presentation, and no epidemiological clue like ticks or tick bites was provided [Reference Estripeaut10, Reference Argüello20]. In previous cases of RMSF in Panama and neighbouring countries, the initial clinical suspicions are mainly dengue or other arboviruses, causing tests for RMSF to be included only after these are negative. Consequently, the delay in diagnosis decreases the probability of a positive prognosis for the patient [Reference de Oliveira3, Reference Argüello20].
Another factor is the sparse knowledge of the epidemiology of RMSF in Panama. Compared to other vector-borne diseases, there is less familiarity about the ecology and distribution of tick vectors. There is confusion about which ticks have been confirmed as vectors (e.g. A. cajennense complex), and other species which can be infected with R. rickettsii, but for which evidence is lacking for their capacity to be vectors of the disease (e.g. H. leporispalustris and D. nitens, see Miller et al. [Reference Miller21]). The epidemiology of TBR and RMSF vary considerably throughout their distribution so local experience and knowledge can be limited. These two new cases occurred in different environmental and ecological conditions.
El Valle de Antón is a rural town with conditions that support different populations of ticks, particularly A. mixtum and R. sanguineus s.l. [Reference Bermudez15, Reference Bermúdez and Miranda22, Reference Bermúdez23]. El Valle and nearby regions have a recent history of circulation of TBR, based on positive serology in domestic mammals [Reference Bermúdez23] and humans [Reference Bermudez15], with evidence of R. rickettsii infecting A. mixtum [Reference Bermúdez13], and confirmed fatal cases of RMSF [Reference Tribaldos11, Reference Bermúdez13]. In rural Panama, A. mixtum is the most anthropophilic species and the main vector of R. rickettsii [Reference Bermúdez13, Reference De Rodaniche16]. In fact, other species in the A. cajennense complex are vectors of R. rickettsii in rural regions of Colombia and Brazil [Reference Tarragona5, Reference Faccini-Martínez24]. Based on these studies and considering the classification proposed by Pinter [Reference Pinter25], this region should be classified as a transmission area for TBR, particularly RMSF.
In contrast, there are no agricultural activities or forested areas close to the home of the second victim. In Panama City, R. sanguineus s.l. is the most common species of tick and has been reported to use humans as hosts, although less frequently than A. mixtum does in rural sites [Reference Bermúdez13, Reference Bermudez15]. Panama City has a history of TBR, starting in 1952 when one case from an urban neighbourhood was linked to the presence of R. sanguineus s.l. In 2007, three new cases of TBR were confirmed in a rural area from this city but could not be linked to a particular species of ticks [Reference Tribaldos11]. Since R. sanguineus s.l. is the clear vector of R. rickettsii in Arizona (the USA) and Mexico [Reference Álvarez-Hernández19, Reference Demma26] and is implicated as a potential vector in urban cases in Costa Rica [Reference Argüello20], our findings of R. rickettsii infecting R. sanguineus s.l. inside the patient's house may be consistent with its role as a vector. The vectorial competence of this species in Panama needs further study given the complexity of the taxonomy of this tick.
Differences in the ecology of the two species of ticks should be considered when establishing control protocols. Amblyomma mixtum is an exophilic species that inhabits paddocks, or stables and horses appear to be its main host, but it can also be found feeding on 20 other species of vertebrates, including humans [Reference Labruna7, Reference Bermúdez13]. In contrast, R. sanguineus s.l. is endophilic and can persist inside houses and on external walls; it maintains a strong parasitic relation to its normal dog host, but can easily adapt to a change of host should canines not be available [Reference Bermúdez13, Reference Álvarez-Hernández19].
Although mosquito-borne diseases such as dengue have a higher prevalence than TBR in both urban and rural Panama, the wide distribution of A. mixtum and R. sanguineus s.l. in Panama needs to be considered with respect to non-specific fevers like TBR. A prompt clinical response to antibiotics such as doxycycline may both benefit the patient and confirm suspicions of a TBR infection even without laboratory confirmation [Reference Minniear and Buckingham2, Reference Alvarez-Hernandez18]. Further research will be needed to evaluate the ecology related to both cases, especially in the urban case given the high prevalence of dogs and R. sanguineus s. l. in many cities in Panama.
Acknowledgement
This study was partially funded by SENACYT Panama Grant FID14-033
We give thanks to Dr Hector Cedeño (MINSA, Panamá) for his help with the initial collection of information; José Oteo (España), Juan Kravcio (HST), JM Pascale (ICGES) and Gregory Dasch (CDC), for their comments on the manuscript and assistance with the English (GD). Alexander Martínez-Caballero is part of the Sistema Nacional de Investigación (SNI) of SENACYT-Panamá.
Author's contribution
SEBC, AM, BM and YZ, conceived the study. AM and SEBC participated in the data collection, analysis and interpretation of data, and drafted the manuscript. GM, MA, JVP and JAS participated in the medical characterisations. GM and JVP participated in the description and analysis of the autopsies and made the pictures presents in the figures. AM, BM, CG, JM-M, YZ and SEBC participated in the conception of the molecular analysis. JBVP, LD and SEBC participated in the field works. All authors participated in the write of the text.
Disclaimers
No conflicts of interest declared by authors.