Introduction
The core target of the 90–90–90 strategy is that at least 73% of people living with HIV (PLWH) have undetectable levels of HIV. The effectiveness of antiretroviral therapy (ART) is critical to achieve this target. Among the associated factors, HIV drug resistance (HIVDR) is increasingly concerning [Reference Gupta1]. In response to the threat of HIVDR, it is necessary to monitor and assess HIVDR with the scaling up of ART. The surveillance of HIVDR in populations initiating ART provides evidence to inform the selection of effective first-line ATR regimens.
During 2014–2016, the associated surveillance showed that the prevalence of pretreatment HIV-1 drug resistance (PDR) reached 10% or above in some low-income and middle-income countries [Reference Tchouwa2–Reference Boender6]. The increased non-nucleotide reverse transcriptase inhibitor (NNRTI) resistance mostly contributed to the increase in PDR [Reference Gupta1]. A systematic review and meta-regression analysis found that the yearly increases in the odds of pretreatment NNRTI resistance were 23% in southern Africa, 17% in eastern Africa and in western and central Africa, and 11% in Asia and in Latin America and the Caribbean [Reference Gupta1]. A recent World Health Organization (WHO) drug resistance report showed that the prevalence of PDR to efavirenz (EFV) and nevirapine (NVP) reached levels above 10% in 12 of 18 countries [7]. Based on the WHO recommendation, when the prevalence of pretreatment NNRTI (EFV and NVP) resistance is ⩾10% and NNRTI-containing regimen is used as first-line ART, a public health response should be initiated, such as using alternative first-line non-NNRTI-based regimens or introducing pretreatment HIVDR testing to guide the selection of first-line ART regimens [8]. To address the significantly increased pretreatment NNRTI resistance in low- and middle-income countries, the WHO updated the guidelines of ART in 2019, in which non-NNRTI-containing regimens were preferentially recommended [9].
Following the global tendency, the prevalence of HIVDR in China is also increasing. The trend of HIVDR in ART-naive individuals increased from 3.75% in 2012 to 6.25% in 2017 and the prevalence of resistance to NNRTIs increased from 1.75% in 2012 to 5.0% in 2017 [Reference Zuo10]. There was yet a whole nationwide survey of PDR in China. However, limited studies have shown that the prevalence of PDR reached or approached the threshold level in some areas, such as 17.4% in Shanghai and 9.9% in Liangshan Prefecture of Sichuan Province [Reference Wang11, Reference Dong12]. These results suggest that region-specific investigations of HIV-1 PDR are necessary.
Yunnan is a southwest frontier province in China and shares a border with Myanmar, Laos and Vietnam. Yunnan is considered a predominant entry point for the HIV-1 epidemic into China and is most hit by HIV-1 [Reference Lu13]. By the end of 2016, the number of PLWH in Yunnan (91 986) ranked second in China, of which 70 577 (76.7%) were receiving ART. Among PLWH receiving ART, the rate of virological suppression was 85.2%. With the scaling up of ART, the effect of PDR on the effectiveness of ART should be considered. Lincang Prefecture boarded Myanmar and was also one of the areas severely affected by HIV-1 in Yunnan. By the end of 2016, the number of PLWH in Lincang Prefecture was 7802. HIV control and prevention in the border area were also impacted by cross-border activities. The overall prevalence of transmitted HIV-1 drug resistance (TDR) remained low in Yunnan Province [Reference Chen14]. However, a recent study found that the occurrence ratio of HIVDR among HIV-1-infected entering travellers from Myanmar into Yunnan was up to 12.8% [Reference Xuan15]. To thoroughly understand the prevalence of PDR in the border area and direct local antiretroviral therapy, surveillance of PDR was carried out in Lincang Prefecture.
Methods
Study participants and sample collection
During 2017–2018, a total of 372 PLWH initiating ART for the first time were enrolled at ART clinics in eight counties of Lincang Prefecture, Yunnan Province. Blood samples were collected before taking antiretroviral drugs and demographic information was collected. Written consent was obtained from all participants. The study was approved by the institutional review board of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention.
Amplification of HIV-1 pol genes
Viral RNA was extracted with QIAsymphony SP (Qiagen GmbH, Hilden, Germany). Partial pol sequences (HXB: 2253–3553) were amplified using in-house nested reverse transcription polymerase chain reaction (PCR). The sequence corresponded to codons 1 to 99 of protease and codons 1 to 299 of reverse transcriptase. The primers and procedures were described in a previous study [Reference Chen16]. PCR products were sequenced by Biomed Co. (Beijing, China) on the ABI 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA).
Phylogenetic analysis
The sequences were assembled using Sequencher 5.1 (Gene Codes, Ann Arbor, MI). ClustalW multiple alignment and manual editing were performed using Bio-Edit 7.0 software. The neighbour-joining phylogenetic tree was constructed using MEGA (Molecular Evolutionary Genetics Analysis, version 5.1) based on the Kimura two-parameter model with 1000 bootstrap replicates.
Drug resistance analysis
Drug resistance mutations (DRM) and levels were analysed by submitting pol sequences to the Stanford HIVdb Program (https://hivdb.stanford.edu/hivdb/by-sequences/). The Stanford algorithm classifies HIVDR into five levels (susceptible, potential low-level, low-level, intermediate or high-level drug resistance) [Reference Liu and Shafer17]. According to the WHO-recommended judgement criteria for PDR, any HIVDR is defined as low-, intermediate- or high-level resistance to the following drugs: two NNRTIs (EFV and NVP), seven nucleotide reverse transcriptase inhibitors (NRTIs, abacavir (ABC), zidovudine (AZT), stavudine (D4T), didanosine (DDI), emtricitabine (FTC), lamivudine (3TC), tenofovir (TDF)) and three proteinase inhibitors (PIs, atazanavir/r (ATV/r), darunavir/r (DRV/r) and lopinavir/r (LPV/r)) [18].
Statistical analysis
Statistical analysis was conducted using the SPSS 19.0 package (SPSS Inc. Chicago, IL). Categorical variables were compared using chi-square analysis or Fisher's exact test as appropriate. Fisher's exact test was used when any expected frequencies were less than five. All tests were two-tailed and a P-value <0.05 was considered significant.
Results
Demographic characteristics of the participants
A total of 372 PLWH were enrolled before initiating ART in Lincang Prefecture during 2017–2018. Of them, 50 samples were not successfully amplified and 322 partial pol gene sequences were obtained. The failure of amplification could be due to viral RNA degradation caused by poor storage and transportation conditions of samples. However, the constituent of the 322 successfully genotyped individuals was not significantly different with that of the total 373 participants (Supplementary Table S1).
Among the individuals with pol sequences, 61.8% (199/322) were male and 38.2% (123/322) were female; the median age was 38 years (range: 15–79 years); 57.1% (184/322) of individuals were of Han ethnicity, while 42.9% (138/322) were minorities. Among them, single, married and divorced/widowed accounted for 25.8% (83/322), 54.7% (176/322) and 19.6% (63/322), respectively. For transmission routes, heterosexual contact, intravenous drug use and homosexual contact accounted for 93.8% (302/322), 4.0% (13/322), 0.9% (3/322), respectively and the remaining 1.2% (4/322) were unknown. The CD4+ T lymphocyte counts were divided into four classes (<200, 200–349, 350–499 and ⩾500 cells/μl), accounting for 29.2% (94/322), 37.0% (119/322), 17.4% (56/322) and 16.5% (53/322), respectively.
The distribution of HIV-1 strain types in the participants
Based on the phylogenetic analysis of pol sequences (Supplementary Figure S1), 11 HIV-1 strain types were detected. Among them, CRF08_BC (70.2%, 226/322) was the predominant strain and the others were URF strains (10.6%, 34/322), CRF01_AE (9.0%, 29/322), CRF07_BC (5.0%, 16/322), subtype B (1.9%, 6/322), subtype C (1.6%, 5/322), CRF55_01B (0.6%, 2/322), CRF59_01B (0.3%, 1/322), CRF65_cpx (0.3%, 1/322), CRF85_BC (0.3%, 1/322) and CRF87_cpx (0.3%, 1/322). URF strains included BC (52.9%, 18/34), CRF01_AE/C (32.4%, 11/34), CRF01_AE/B (11.8%, 4/34) and CRF01_AE/BC (2.9%, 1/34).
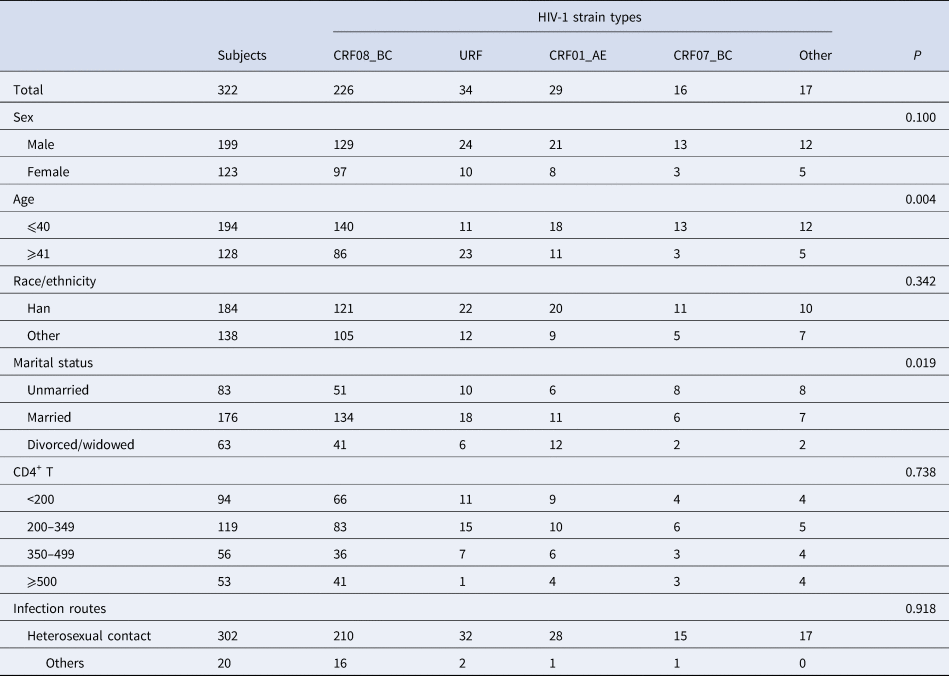
The distributions of HIV-1 strain types were not significantly different by sex, race/ethnicity, CD4+ T lymphocyte count or transmission route (Table 1). However, the distributions of HIV-1 strain types by age and marital status showed differences. The proportion of URF strains in the subgroup aged ⩾41 years was higher than that in the subgroup aged ⩽40 years. In the subgroups divided by marital status, the proportion of CRF08_BC was higher among the married and the proportion of CRF01_AE was higher among the divorced/widowed.
Table 1. Demographic characteristics and the distribution of HIV-1 strain types
The DRM among the participants
Among the 322 participants, 34.2% (110/322) harboured drug resistance-associated mutations. In detail, 3.7% (12/322) harboured NRTI resistance-associated mutations, 31.6% (102/322) harboured NNRTI resistance-associated mutations and 2.8% (9/322) harboured PI resistance-associated mutations.
The drug resistance-associated mutations are shown in Table 2, among which E138A/G/K/R (14.3%, 46/322) and V179E/D/T (13.7%, 47/322) were far higher than the others. The proportion of E138 mutations among CRF08_BC (19.9%, 45/226) was higher than that in the other HIV-1 strain types (1.0%, 1/96; χ 2 = 19.593, P < 0.001).
Table 2. The frequency of HIV-1 drug resistance mutations among the participants
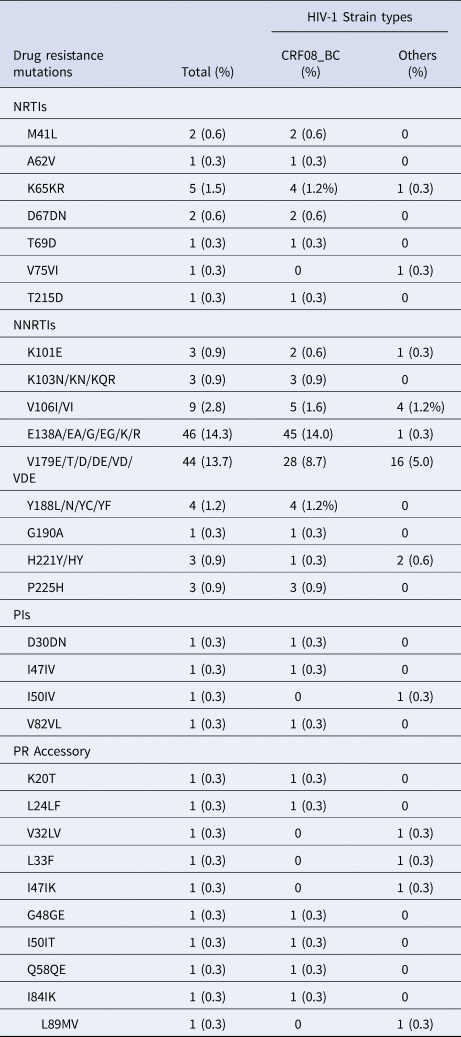
Among the DRMs detected, some key DRMs can independently confer resistance (Supplementary Table S2). Among the key DRMs for NRTIs, K65R (1.6%, 5/322) conferred high-level resistance, T69D (0.3%, 1/322) conferred intermediate resistance and M41L (0.6%, 2/322), D67N (0.6%, 2/322) and T215D (0.3%, 1/322) conferred low-level resistance. Among the key DRMs for NNRTIs, Y188C/F/L (0.9%, 3/322), K103N (0.6%, 2/322) and G190A (0.3%, 1/322) conferred high-level resistance, K101E (0.9%, 3/322) and P225H (0.9%, 3/322) conferred intermediate resistance and H221Y (0.9%, 3/322) conferred low-level resistance. Among the key DRMs for PIs, I50 V (0.3%, 1/322) conferred intermediate resistance and I47 V (0.3%, 1/322) conferred low-level resistance. Although E138 G and V179D/E independently confer potential low-level resistance to EFV/NVP, their combination (LC17S374 and LC17S467) resulted in low-level resistance to EFV/NVP (Supplementary Table S2).
The characteristics of PDR among the participants
According to WHO-recommended judgement criteria for PDR [18], 7.5% (24/322, 95% CI: 4.6–10.3%) of the sequences were HIV-1 drug-resistant strains. The prevalence of resistance to NRTIs, NNRTIs and PIs was 3.1% (10/322, 95% CI: 1.2–5.0%), 5.0% (16/322, 95% CI: 2.6–7.4%) and 0.6% (2/322, 95% CI: 0–1.5%), respectively.
The resistance levels to the 12 antiretroviral drugs were further analysed (Fig. 1). The frequencies of resistance to seven NRTIs ranged from 1.2% (4/332) to 2.8% (9/332), among which high-level resistance to D4T, DDI and TDF was detected, whose frequencies were all 1.6% (5/322). However, the frequencies of NNRTI resistance were higher. The frequencies of resistance to EFV and NVP were 4.3% (14/332) and 5.0% (16/322), respectively. The proportions of high-level resistance to EFV and NVP were both 1.9% (6/332). Except for ATV/r, resistance to DRV/r and LPV/r was detected and the frequencies ranged from 0.3% (1/322) and 0.6% (2/322), respectively.
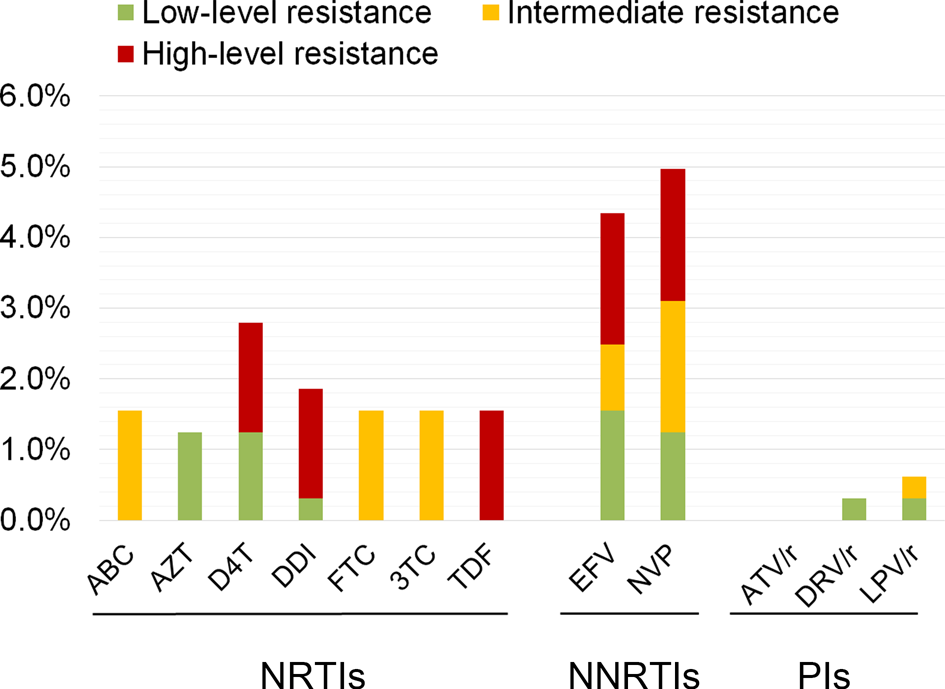
Fig. 1. The proportion of pretreatment HIVDR to 12 antiretroviral drugs listed in the WHO surveillance guidelines of pretreatment drug resistance among 322 PLWH initiating ART for the first time in Lincang Prefecture of Yunnan Province.
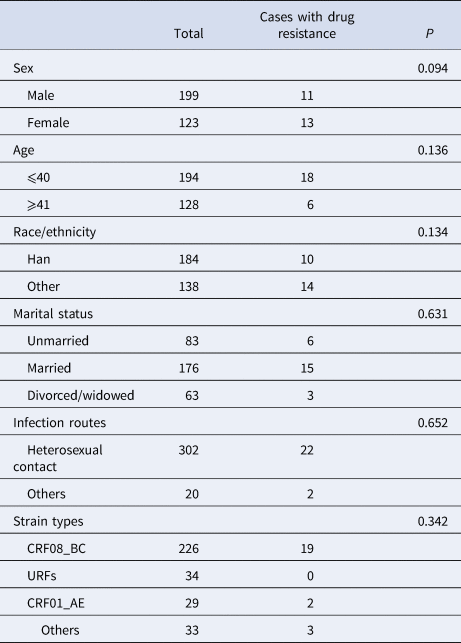
The distributions of drug-resistant strains showed no difference by sex, age, race/ethnicity, marital status, transmission route or HIV-1 strain type (Table 3).
Table 3. The distribution of pretreatment HIVDR among the participants
Discussion
In this study, the characteristics of HIV-1 strain types and PDR were analysed among the individuals initiating ART in Lincang Prefecture, Yunnan Province.
Similar to the epidemic trend of HIV-1 strain types in Yunnan [Reference Chen14], HIV-1 genetics have become more complicated in Lincang. More than ten HIV-1 strain types were found among the participants. Some CRF strains were found in Lincang for the first time, including CRF55_01B, CRF59_01B, CRF65_cpx, CRF85_BC and CRF87_cpx. These CRF strains were first identified in the other part of Yunnan or outside Yunnan [Reference Miao19–Reference Feng24]. This suggested that the intervention of the mobile population should be considered.
Strikingly, the proportion of URF strains increased to second place, which contributed to the HIV-1 genetic diversity in this area. In particular, the proportion of URF strains in the subgroup aged ⩾41 years was higher than that in the subgroup aged ⩽40 years, which suggested that complex transmission-associated factors could exist in the subpopulation of elderly persons. In Yunnan, the annual reported cases aged above 50 years continued to increase and the proportion increased from 8.9% in 2011 to 21.3% in 2017. Thus, HIV control and prevention in this subpopulation should be considered. In addition, the distribution of HIV-1 strain types by marital status showed significant differences, which suggested that different transmission patterns might exist in sexual transmissions, such as commercial and noncommercial sexual behaviours. Despite the lack of direct behaviour data, the higher proportion of CRF01_AE among the divorced/widowed might be associated with commercial sexual behaviour because CRF01_AE was first found among female sex workers [Reference Cheng25] and was the preponderant strain in this subpopulation in the early stage of the HIV-1 epidemic in Yunnan [Reference Lu13, Reference Zhang26]. However, further investigation is needed.
Among the participants, 31.6% (102/322) carried NNRTI resistance-associated mutations, which was far higher than the proportions of NRTI and PI resistance-associated mutations. Since free ART was initiated in China in 2003, NNRTI-based regimens have been the first-line regimens, in which NNRTIs included only NVP and EFV. However, NNRTIs have a low genetic barrier to resistance, whereby a single mutation in the binding site may confer resistance to one or more NNRTIs [Reference Mackie and Geretti27]. In addition, most NNRTIs have a long plasmatic half-life [Reference Usach, Melis and Peris28]. When NNRTI-based regimens were used, poor adherence could result in a single dose status of NNRTIs, whereby NNRTI-resistant mutants are easy to select [Reference Mackie and Geretti27]. Furthermore, these DRM have little effect on the replication adaptability of HIV-1 [Reference Usach, Melis and Peris28]. Thus, these resistance mutations accumulated over time. Worldwide, NNRTI resistance is a prominent phenomenon and shows a similar rising trend [Reference Gupta1]. Thus, the WHO selects the prevalence of NNRTI resistance as the threshold to initiate the public health response.
Among the NNRTI resistance mutations in this study, the key mutations were K103N, Y188L and G190A, which usually cause high-level resistance to NNRTIs but whose frequencies were low among the participants. However, the predominant NNRTI-associated mutations were E138 and V179, accounting for 14.3% and 13.7%, respectively. E138A/G/R results in low-level resistance to RPV and E138K results in intermediate-level resistance to RPV. However, V179 mutations result in potential low-level resistance when existing alone as accessory mutations to regulate the effect of other NNRTI mutations. Furthermore, E138 seemed to be specific to the HIV-1 strain type. The frequency of E138 mutations was higher in CRF08_BC. E138 mutations have low- or intermediate-level resistance to rilpivirine (RPV) but not to EFV, NVP, doravirine (DOR) or etravirine (ETR). In addition, the frequencies of NRTI and PI resistance mutations were lower than those of NNRTI resistance mutations. Among the detected NRTI resistance mutations, K65R is a key mutation that causes intermediate/high-level resistance to TDF, DDI, ABC and d4T and low/intermediate resistance to 3TC and FTC.
The overall PDR level in western Yunnan (7.5%) was higher than the average level in Asia (3.8%) [Reference Gupta1]. However, it was lower than the prevalence among HIV-1-infected entering travellers from Myanmar into Yunnan (12.8%) [Reference Xuan15]. The HIVDR showed a tendency to increase over time. In 2012, the TDR still had a low prevalence level in Lincang (<5%) [Reference Chen29]. In the whole nation, the rate of TDR remained relatively low (3.0%, 95% CI: 2.8%–3.2%) [Reference Zuo10, Reference Zhao30] but showed a specific regional pattern. The prevalence of TDR was higher in Central China than in South and North China [Reference Zuo10]. Similarly, the prevalence of PDR also displayed regional specificity. The prevalence detected in western Yunnan appeared lower than those reported in China, such as 17.4% in Shanghai and 9.9% in Liangshan Prefecture of Sichuan Province [Reference Wang11, Reference Liu31].
Although RPV was not one of the surveillance drugs in the WHO-recommended judgement criteria of PDR, 40 patients were resistant to RPV but not EFV and NVP. As mentioned above, these cases were characterised as CRF08_BC carrying E138 mutations. Recently, RPV was removed from the draft list of the 1st batch of encouraged generic drugs issued by the China National Health Commission [32]. The present study provided information to support this decision. At least, RPV is not suitable for use in the CRF08_BC highly circulating areas. Thus, the association of drug resistance with HIV-1 strain types should be evaluated. In addition to genetic drug resistance, the clinical significance of HIV-1 strain type-specific mutations should be further evaluated.
In China, the National Free ART Program is the main body of antiretroviral therapy. The first-line regimens of free ART combine two NRTIs and one NNRTI. However, the kinds of free ART drugs are limited, including four NRTIs (AZT, 3TC, TDF and ABC), two NNRTIs (EFV and NVP) and one PI (LPV/r). In addition, the scale of patients receiving ART is increasing. It is still a challenge to control HIVDR increases. According to the threshold for triggering a public health response for pretreatment drug resistance to NNRTIs, the alteration of the first-line ART regimens was not yet supported by the result of the present drug resistance surveillance. However, the surveillance of PDR should be regularly carried out to evaluate the effectiveness of first-line regimens. Presently, pretreatment HIVDR testing is not a routine test in the National Free ART Program and it is important to monitor viral load and timely determine the occurrence of drug resistance during ART. According to the WHO update of recommendations on first- and second-line antiretroviral regimens in 2019 [9], dolutegravir (DTG)-containing regimens are recommended as the preferred first-line regimens for adults, adolescents and children and the raltegravir (RAL)-containing regimen is recommended as the preferred first-line regimen for neonates. Although these two kinds of integrase strand transfer inhibitors (INSTIs) are available in China, they are still imported drugs and need to be paid by patients. Thus, it will maximise the benefits of public health to maintain the effectiveness of the current regimens. In addition to being used as a treatment, antiretroviral drugs are widely used to prevent HIV-1 infection. The scaled-up use of antiretroviral drugs as prevention, such as the promotion of the prevention of mother-to-child transmission programmes, pre-exposure prophylaxis and post-exposure prophylaxis, would increase the occurrences of HIVDR, which should be considered.
In conclusion, this study disclosed the characteristics of HIV-1 molecular epidemiology in one area severely affected by HIV-1 in Yunnan. HIV-1 genetics is becoming diverse and complex. The prevalence of PDR remained under the threshold of initiating a public health response. However, it is necessary to strengthen drug resistance surveillance and viral load testing to determine the occurrence of drug resistance during ART in a timely manner.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S095026882000093X.
Acknowledgements
We acknowledge the staff at the local Centre for Disease Control and Prevention for assistance in coordinating sample collection.
Financial support
This work was supported by the Major Project of China's ‘Thirteen Five-year Plan’ for Science and Technology Development (2018ZX10715006) and the Yunnan Science and Technology Talent and Platform Program (2019HB053).
Conflict of interest
None.






