INTRODUCTION
The unwanted and unwelcome presence of Streptococcus pneumoniae in otherwise sterile body compartments such as the blood and cerebrospinal fluid manifests itself as invasive pneumococcal disease (IPD) [1]. Other capsulated organisms such as Neisseria meningitidis and Haemophilus influenzae type b also cause invasive diseases such as septicaemia, meningitis and septic arthritis [Reference Apicella, Mandell, Douglas, Bennett, Dolin and Kelvin2, Reference Dominguez3]. However, it is the often insidious onset of IPD, the pre-emptive use of antibiotics before bacteriological culture and the reluctance to perform lumbar puncture in the face of neurological signs that make accurate ascertainment of IPD incidence difficult [Reference Leeming4, Reference McIntyre5]. Surveillance has been suboptimal due to either an absence of national networks or the use of sentinel sites that may not accurately reflect the national burden [Reference Cornaglia6]. Now that there is an intervention strategy in the form of pneumococcal conjugate vaccine (PCV), it is even more important to institute optimal reporting and surveillance systems for IPD, in order that an accurate measure can be obtained of its impact. Vaccine manufacturers have European networks that can be harnessed to perform, and to obtain the results of, studies designed to measure the local burden of disease. The purpose of this paper is to document the burden of IPD in different European countries before the widespread use of 7-valent PCV.
METHODOLOGY
Medical personnel in Wyeth affiliates across Europe, covering the EU25, Norway, and Switzerland, were asked to provide recent published and unpublished data for IPD by country, before the widespread use of 7-valent PCV. Specifically, we sought data on reporting period, data source, patient age range, definition of IPD, type of IPD, actual numbers with IPD, and age-specific rates of IPD. Serotype and/or serogroup coverage for 7-valent PCV was requested. Mortality data was not specifically sought, although we have noted it when available; data on sequelae was not specifically sought. Recent literature was also searched to provide as wide a representation as possible. This included OVID and abstracts from the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), European Society for Paediatric Infectious Diseases (ESPID), the International Symposium on Pneumococci and Pneumococcal Disease (ISPPD), and the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), dating from 2000.
Some national reference centres display a website. For example, the Czech Republic (Czech Paediatric Society; http://www.szu.cz/cema/epidat/epidat.htm and http://www.ockovanideti.cz/), Denmark (Statens Serum Institute; www.ssi.dk), England and Wales (http://www.hpa.org.uk/infections/topics_az/pneumococcal/data.htm), Scotland (http://www.show.scot.nhs.uk/scieh/), Germany (http://www.pneumococcus.de/), The Netherlands (http://www.pneumokokken.nl/), and Portugal (http://www.insarj.pt/).
These national websites were also searched. The Czech, Danish, Dutch and Portuguese sites are not yet populated with national data on IPD; the England/Wales/Scotland and German sites were used in obtaining material for the present analysis. There are no national pneumococcal websites in Austria, Belgium, Finland, France, Greece, Hungary, Italy, Norway, Slovenia, Spain, Sweden or Switzerland. (A Nordic pneumococcal website is planned.) The Portuguese site displays information on pneumococcal resistance. Links to national sites are available through: http://www.eurosurveillance.org/links/links-02.asp
RESULTS
The results are shown in Tables 1–5.
Clinical and microbiological definitions of IPD
The local clinical and microbiological definitions by country, where available, are detailed in the Appendix. The definitions of IPD included ‘isolation of Streptococcus pneumoniae from a normally sterile body site’. However, in varying degrees, microbiological diagnostic methods (culture, polymerase chain reaction, latex agglutination) and clinical entities (sometimes including pneumonia) were both part of the definitions in use in some countries.
Published sources of data
Table 1 shows the published sources of data and reporting periods for paediatric IPD in Europe. The reporting periods reflect reported rates before the widespread use of 7-valent PCV in Europe. Representatives of 27 countries were approached and there were responses from 18. IPD incidence rates were obtained from the following countries: Austria, Belgium, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Italy, The Netherlands, Norway, Portugal, Slovenia, Spain, Sweden, Switzerland and the United Kingdom.
Table 1. Published sources of data and reporting periods for paediatric IPD in Europe

Unpublished sources of data
In addition to the published studies, unpublished data is also available. Table 2 shows unpublished, primary source data for paediatric IPD in Europe. For Hungary, an unpublished recent Wyeth-sponsored study entitled ‘Prospective evaluation of the incidence of IPD (invasive pneumococcal disease), the serotypes and the resistance against commonly used antibiotics of S. pneumoniae isolates in hospitalized children up to 5 years of age with IPD including meningitis from selected paediatric wards (covering approximately 90%) in Hungary: a two year observational study’, reported 55 infants and children with IPD.
Table 2. Unpublished, primary source data for paediatric IPD in Europe

Unpublished data is also shown for Italy, The Netherlands and Portugal. Although information on suspected pneumococcal pneumonia was not specifically sought, pneumococcal pneumonia requiring hospitalization in Portugal was reported to occur at a rate of 144·44/100 000 in those aged 0–2 years and 80·62 in those aged 0–4 years [Reference Serrano, Ramirez and Melo-Cristino43].
Reported rates of IPD by age and country
Table 3 shows the reported rates per 100 000 of total IPD (including pneumococcal meningitis) and of pneumococcal meningitis by age (<1 year and <2 years) by country. Table 4 shows the reported rates per 100 000 of total IPD (including pneumococcal meningitis) and pneumococcal meningitis by age (2–5 years and <5 years) and by country.
Table 3. Reported IPD incidence rates* by country for infants and children aged <2 years

* Reported incidence per 100 000 population per year.
Table 4. Reported IPD incidence rates* by country for children in the 2–5 and <5 years age groups

* Reported incidence per 100 000 population per year.
Serotype coverage
Table 5 shows the published rates of 7-valent PCV serotype coverage by age and by country.
Table 5. Summary of published serotype coverage data for total IPD by country
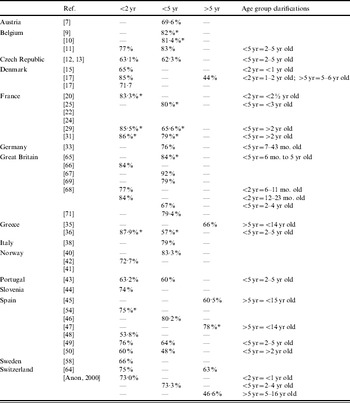
* Serogroup coverage.
Regional differences
For Spain, there are reported to be regional differences. One report suggests that Spain may have the highest reported rate of IPD in Europe at 174/100 000 for the 0–23 months age group [Reference Perez and Solis49]. Another report shows that in Navarre and the Basque country the reported rate for this age group is 93·5/100 000, while that for children aged 2–5 years is 30·1/100 000 (Navarre 45·5, Basque Country 25·4) [Reference Bernaola52]. Similarly, for meningitis in the 2–5 years age group the overall reported rate in Spain is 1·5/100 000, while in Catalonia, it is 2·5 and in Galicia, Basque Country and Navarre it is reported to be 0·0 [Reference Casado53]. In Valencia, with prospective active surveillance at 19 regional hospitals, the rates for all IPD were lower than for the rest of Spain at 16·8/100 000 for those aged ⩽2 years and 10·5/100 000 for those aged ⩽5 years. Regional differences are also reported in Germany, and are probably related to day-care attendance [Reference Von Kries33, Reference Siedler34].
DISCUSSION
Not only are the definitions of what constitutes IPD in various European countries different, but also reported incidence rates and potential 7-valent PCV coverage vary widely. Our approach to compiling the data, while not systematic, does draw on a variety of sources and offers insights into why the differences exist. It is possible that responsible authorities in certain countries have not published their data or data has been published selectively. By contrast, sentinel sites across the United States may provide a more accurate picture of the burden of IPD there.
Regional and local differences in the reported incidence of IPD in Europe are exemplified by Spain. Spain also tops the ‘league table’ for IPD in the <2 years age group with reported rates between 93·5 and 174/100 000, depending on the source. This compares with the reported rate of IPD of 166·9/100 000 child-years in the United States before the introduction of 7-valent PCV [Reference Robinson73] but is still very much less than the very high reported rate of 2052/100 000 reported for infants and children aged <2 years in central Australia [Reference Torzillo74]. These are likely to be due to real differences in pneumococcal carriage, transmission, exposure and susceptibility.
There is a lack of consistency by which IPD is reported by particular age bands, making comparisons difficult, as can be seen from Tables 3 and 4. In most reporting systems, pneumococcal meningitis can be distinguished from overall IPD. The highest reported rates for pneumococcal meningitis are in the <12 months age group in Spain (17·75/100 000) [Reference Casado53] and in those aged <2 years in Belgium (16·1/100 000) [Reference Vergison11]. These are considerably higher than rates reported by Noah and Henderson in a survey of bacterial meningitis in Europe during 1999/2000 in which the reported crude incidence for combined laboratory-confirmed and notified cases of pneumococcal meningitis ranged from 0·07/100 000 per year for Poland to 1·06/100 000 per year for The Netherlands [Reference Noah and Henderson75]. In the present analysis, while information on the sequelae of pneumococcal meningitis was not specifically sought, sequelae were noted to occur in up to 20% of cases of pneumococcal meningitis in Sweden [Reference Eriksson, Henriques and Ekdahl57] and 30% in France [Reference Olivier22], reflecting not only a preventable health burden but also a life of considerable disability for survivors.
Whilst we did not specifically seek data on suspected pneumococcal pneumonia, it was provided on occasions as part of IPD reporting. For example, pneumococcal pneumonia requiring hospitalization in Portugal was reported to occur at a rate of 144·44/100 000 in those aged 0–2 years and 80·62 in those aged 0–4 years [INFARMED, see Table 2], reflecting a considerable burden on health-care utilization. Bacteraemic pneumonia was also included in some of the IPD reporting from Greece and Italy.
Where potential 7-valent PCV serotype coverage data was available, there was a range from 48% to 85% depending on the age group and the clinical presentation, although in general the coverage was >60% (Table 5). High potential coverage for infants and young children was evident despite variations between countries in the distribution of individual serotypes to coverage. Not only do vaccination schedules vary between countries (e.g. 2, 3, 4, 12–15 months vs. 2, 4, 6, 12–15 months) but also recommendations for the use of 7-valent PCV vary about which at-risk group should be targeted [Reference Fletcher76]. Furthermore, what is perceived as being a condition predisposing to IPD differs between countries. For instance, cochlear implant, diabetes mellitus, previous IPD, prematurity, low birth weight, failure to thrive or day-care attendance are not universally perceived as being risk factors for IPD. Finally, the 23-valent pneumococcal polysaccharide vaccine shows poor efficacy in the very young. Although this vaccine is recommended for individuals aged >2 years with certain comorbidities, it is unlikely to be used in healthy infants and children [Reference Finn77, Reference Finn78].
With the expansion of the EU, the development of a Europe-wide reporting system for serious infections such as IPD, which is similar to the Active Bacterial Core Surveillance/Emerging Infections Program Network of the US Centers for Disease Control and Prevention, will be useful for coordinating prevention strategies, and evaluating control measures including vaccination under the aegis of the European Centre for Disease Prevention and Control (http://www.ecdc.eu.int/). Furthermore, given the emergence of pneumococcal antibiotic resistance, the coordination of prevention efforts has wide implications for minimizing the spread of resistant clones from one country to another. There is already a European network for monitoring the emergence of pneumococcal antibiotic resistance called the European Antimicrobial Resistance Surveillance System (EARSS), which can be found at: http://www.earss.rivm.nl/PAGINA/interwebsite/home_earss.html.
What are the chances of coordinating data collection efforts across Europe? For organisms such as S. pneumoniae, where disease affects all age groups and clinical manifestations are legion, the logistics are formidable. With the early administration of antibiotics, highly sensitive tests such as the polymerase chain reaction may be the only method reliable enough to detect the organism [Reference Sheppard79]. In the meantime, the spread of antibiotic non-susceptible strains may only become apparent through the emergence of breakthrough disease [Reference Cartwright80, Reference Das81] unless European surveillance programmes are in place. As an example, the Tracking Resistance in the United States Today (TRUST) programme reported increasing rates of pneumococcal resistance over the 4-year period 1998–2002, before the widespread use of 7-valent PCV in the United States [Reference Karlowsky82]. Organizations such as The Committee of the European Union Network for the Surveillance and Control of Communicable Diseases, the European Society for Paediatric Infectious Diseases, and Eurosurveillance (see: www.eurosurveillance.org) have the potential to coordinate efforts for defining the burden of serious infections such as those caused by S. pneumoniae. The EU has established a series of standard case definitions for infectious diseases, including pneumococcal infections, at this website. With respect to prevention, a report by the Summits of Independent European Vaccination Experts has stated: ‘Only if the European Union comes up with and implements common vaccination goals with firm deadlines can the best health through vaccination of all Europeans be accomplished’ [Reference Schmitt83].
The burden of paediatric IPD in Europe is considerable, even though the reported incidences vary 100-fold. Nonetheless, it remains difficult to obtain the actual rates for some countries. Under reporting, differences in reporting methods, antibiotic prescribing and disparities in blood-culturing practices may distort the true picture, although real differences do exist due to variability in pneumococcal carriage, transmission, exposure and susceptibility. A standardized approach not only to surveillance and reporting but also to diagnosis and management across Europe is needed. This is the central dilemma of the European concept – the preservation of treasured cultural differences while attaining standardization in areas where comparison is required. In conclusion, PCV has the potential to prevent, once recognized, much of the pneumococcal infection morbidity and mortality arising from Europe.
APPENDIX. Local clinical and microbiological definitions of IPD by country
Austria: ‘Cases were eligible for evaluation if they had been admitted to a paediatric hospital and if Streptococcus pneumoniae was identified by culture, polymerase chain reaction (PCR), or a latex agglutination test of blood, cerebral spinal fluid (CSF), or any other normally sterile site. Case identification was based on two independent surveillance systems – one hospital-based and the other laboratory-based’ [Reference Rendi-Wagner7].
Belgium: ‘Six clinical entities were defined as IPD: meningitis, pneumonia with positive blood culture, pneumonia with empyema, clinical septicaemia, bacteraemia without focal lesion (including cases with acute otitis media) and other (arthritis, peritonitis). Strains of Streptococcus pneumoniae isolated from a normally sterile site in a child less than 5 years old were sent by the local laboratories to the National Reference Laboratory where serotyping and antibiotic testing (E-test) were performed. To be eligible, each patient had to meet the following inclusion criteria: (1) be younger than 5 years, (2) have a Streptococcus pneumoniae isolated from at least one culture of a normally sterile site like blood, CSF, pleura, peritoneal or articular fluid and (3) obtain written informed consent from parents or legal guardian’ [Reference Vergison11].
Czech Republic: ‘Clinical specimens (blood, cerebrospinal fluid, autopsy specimens, lung puncture aspirate, bronchoalveolar lavage fluid, sputum) of patients with invasive pneumococcal infections’ [Reference Motlova13].
Denmark: ‘Streptococcus pneumoniae isolates from normally sterile body sites … Included in the present study are invasive isolates – either from blood or cerebrospinal fluid (CSF) … Only one isolate per patient obtained during a 30-day period is included. When both a CSF and a blood isolate were received from the same patient with meningitis, only, the CSF isolate was included’ [Reference Kaltoft, Zeuthen and Konradsen15, Reference Kaltoft, Madsen and Konradsen17].
Finland: ‘A network of all microbiologic laboratories and paediatric wards in Finland. Children aged 0 to 15 years who were admitted to a hospital with S. pneumoniae isolated from blood, cerebrospinal fluid, or deep aspirate sample’ [Reference Eskola18, Reference Klemets19].
France: ‘Clinical and bacteriological data related to pneumococcal infections from hospitals located throughout the entire country’ [Reference Mehl-Auget21–Reference Laurichesse26Reference Mehl-Auget21–Reference Laurichesse26, Reference Floret30, Reference Aujard31].
Germany: ‘Patients were enrolled in the study if they had been admitted to a paediatric hospital and if S. pneumoniae had been isolated from at least 1 culture of blood, CSF, or a sample from any other normally sterile body site. Isolates from middle ear fluid were not included’ [Reference Von Kries32–Reference Siedler34].
Greece: ‘The medical records of patients who had as discharge diagnoses bacteraemia, bacterial meningitis, or lobar pneumonia and the medical records of children from whom S. pneumoniae had been isolated from blood, cerebrospinal fluid (CSF), or any other normally sterile body site were reviewed and abstracted’ [Reference Syriopoulou35]. The results included cases of probable pneumococcal pneumonia.
Hungary: ‘Severe cases of invasive pneumococcal disease which are hospitalised in Hungarian children's wards’ [unpublished study].
Italy: ‘Positive culture for S. pneumoniae in normally sterile sites, as blood … blood culture performed for the following: infection site clinically absent (fever peak >38·5°C or two close febrile episodes >38°C, and neutrophilia (>15 000/ml); clinically diagnosed pneumonia; active broncho-pneumonia site; post-surgical infectious episode; febrile episode in surgical and/or neutropenic patients’ (Professor Schito, personal communication). Blood or CSF positive by culture or PCR [Reference D'Ancona38].
The Netherlands: ‘All clinical microbiological laboratories send their CSF to The Netherlands Reference Laboratory for Bacterial Meningitis (NRBM)’, ‘pneumococci isolated from non-meningitis patients with bacteraemia were submitted to NRBM’ [Reference Spanjaard39].
Norway: ‘Patients included in the database are those infected with Streptococcus pneumoniae, from whom positive specimens have been obtained from a sterile location – usually blood, cerebrospinal fluid (CSF), or both’ [Reference Pedersen41, Reference Hasseltvedt and Høiby42].
Portugal: ‘614 consecutive S. pneumoniae were isolated from different specimens (blood, CSF, pleural liquid) at 26 hospitals in Portugal’ [Reference Serrano, Ramirez and Melo-Cristino43].
Slovenia: ‘All invasive strains … from primary sterile body sites’ [Reference Paragi44].
Spain: ‘Isolation of S. pneumoniae in blood, cerebrospinal fluid or any other sterile biological fluid’ [Reference Perez and Solis49, Reference Díez-Domingo51–Reference Rodriguez-Creixems54]. ‘Isolation of Streptococcus pneumoniae and Hib in blood, cerebrospinal fluid (CSF) and synovial fluid’ [Reference Villo Sirerol and Blanco48].
Sweden: ‘Isolates obtained from blood or CSF’ [Reference Eriksson, Henriques and Ekdahl57, Reference Hedlund58].
Switzerland: ‘All isolated pneumococcal strains found in sterile places (i.e. cerebrospinal fluid, blood, etc.) are analysed’ [Reference Venetz, Schopfer and Mühlemann59–Reference Bundesamt64].
United Kingdom: ‘All clinically significant bacterial isolates from blood, cerebrospinal fluid (CSF) and other sterile sites’ [Reference Kyaw66, Reference Miller68, Reference George and Melegaro70–Reference George and Melegaro72]. ‘Isolation of S. pneumoniae from blood, cerebrospinal fluid (CSF), or other normally sterile body sites’ [Reference Ispahani65].
ACKNOWLEDGEMENTS
The authors are grateful to the following for providing information: Ralf Lenhard, Markus Mattauch and Jacek Nowak (Wyeth, Austria and Hungary), Prof. Herwig Kollaritsch, Prof. Ingomar Mutz, Prof. Apostolos Georgopoulos, Prof. Michael Kundi (Austria); Mark Baelus and Sophie Leyman (Wyeth, Belgium); Martin Myrdacz (Czech Republic); Maja Schrøder (Wyeth, Denmark); Steffen Glismann and Jens Jørgen Christensen (Denmark); Julia Krisch, Ralf Sprenger and Petra Venker (Wyeth, Germany), Nick Zissis, Dimitris Kiatos, Vasso Karamanou and Gregory Skandamis (Wyeth, Greece) and Prof. V. Syriopoulou (Athens Agia Sophia Hospital), Prof. D. Kafetzis (Athens Aglaia Kyriakou Hospital), Prof. K. Malaka-Zafiriou (Thessalonika Ippokration Hospital), Dr Tsilimingaki (Crete Venizelion Hospital), Prof. S. Sbyrakis and A/Prof. E. Galanakis (Crete University Hospital Iraklion), Dr I. Tsangaropoulou (Thessalonika Infectious Diseases Hospital) (Greece); Ciro Bianchi, Serena Zucchetta and Alessandro Zollo (Wyeth, Italy); Lodewijk Spanjaard (The Netherlands); Jolanda Crombach (Wyeth, The Netherlands); Steffen Ristun (Wyeth, Norway); Manuela Caniça and Mario Ramirez (Portugal); Jose Brandao, Ligia Nunes, Eduardo Ribeiro and Nuno Silverio (Wyeth, Portugal); Moe Kyaw (Scotland/CDC); Désiré Galvez, Consuelo Nicolas and Ana Perez (Wyeth, Spain); Ilona Idanpaan-Heikkila, Vesa Loponen, Stefan Söder, Mikael Sörberg (Wyeth, Finland, Sweden); Petri Ruutu (Finland); Timo Imbach (Wyeth, Switzerland).
Thanks are also due to Alessia Melegaro from the Modelling and Economics Unit, Communicable Disease Surveillance Centre, Health Protection Agency, Colindale, UK, for helpful comments on the manuscript.
DECLARATION OF INTEREST
The authors are employed by Wyeth, which owns and markets 7-valent pneumococcal conjugate vaccine.







