INTRODUCTION
The global burden of shigellosis includes 165 million episodes annually and more than 1 million deaths, about 99% of which are in developing countries, where Shigella dysenteriae, S. boydii, S. flexneri and S. sonnei share the responsibility as aetiological agents [Reference Kotloff1]. In developed areas, S. sonnei has been the predominant infectious agent causing at least 70% of the infections for the last two decades [Reference Kotloff1, Reference Gupta2]. In most of the developed countries the annual incidence rates range between 5 and 10/100 000 [Reference Kotloff1], although some locations with low-income minorities may have much higher incidence rates [Reference Hayes-Bautista3, Reference Lee4]. In 1998, Sobel et al. [Reference Sobel5] described an outbreak of shigellosis in a community of observant Jews in Brooklyn. It was noted that clonally related strains of S. sonnei caused similar outbreaks simultaneously in distant communities of observant Jews. The authors attributed the spread of disease to travels by visiting relatives. Risk factors for spread of shigellosis in an observant Jewish community in New York included day-care attendance (OR 16·1) and age <5 years (OR of 6·3) [Reference Garrett6].
Bene Beraq (BB), a suburban city in the Tel Aviv district (population 139 000), has one of the largest observant (ultra-orthodox) Jewish communities in the world. The city is characterized by low income and a high population density. Due to a high fertility rate, about 14·5% of the city's population consist of children aged <5 years, which is about twice the proportion in other cities in the district. These conditions make the BB population highly susceptible to shigellosis.
The aim of the study is to describe patterns of shigellosis in the observant community of BB and the community-based educational interventions that took place following outbreaks.
METHODS
Surveillance data
Data on incidence of shigellosis in BB were obtained from the Tel Aviv Health District Authority that runs a passive surveillance system, part of the notifiable diseases surveillance of the Israeli Ministry of Health's epidemiology department. Notifications on individual patients diagnosed with shigellosis are received from doctors, clinics, hospitals and laboratories [7] and entered into a computerized system. We retrieved summaries of these notifications from 1 January 1998 to 31 December 2006.
Data from Ma'aynei Hayeshua Medical Centre (MHMC)
MHMC is a 224-bed community-based hospital. The population served by the hospital is observant and includes residents of BB as well as individuals from other observant communities. The Paediatrics Department in MHMC was established in February 2000.
Interventions
MHMC planned and carried out educational interventions that were initiated when the number of shigellosis cases soared in the years 2002, 2004, and 2006; each of the interventions lasted for 2–3 months. These interventions were performed in active cooperation with the authorities of the Tel Aviv Health District and the BB municipal Department of Education. In all three interventions the same means were practised: articles were published in orthodox dailies and weeklies; posters were displayed in the main streets of BB; pamphlets of supporting declarations from religious authorities were distributed in synagogues; and caregivers of private and public day-care centres were encouraged to attend evening lectures about shigellosis and prevention of the infection. The main message was that shigellosis dysentery is preventable. It should be noted that rituals in this population include frequent hand washing, but these do not require the use of soap. Detailed guidelines for hygiene and sanitation including obligatory hand washing with soap before and after feeding and cleansing children were distributed. It was emphasized that children with diarrhoea must not visit their care institution before full recovery.
LABORATORY EVALUATION
Microbiological methods
In the MHMC laboratory, all faecal samples were routinely examined for Salmonella spp., Shigella spp., enteropathogenic Escherichia coli and Campylobacter spp. using accepted technology [Reference Koneman8]. Appropriate isolates were tested for agglutination with Shigella species-specific antisera (Denka Seiken Co. Ltd, Japan). Susceptibility was studied by disk diffusion, according to Clinical and Laboratory Standard Institute (CLSI) protocol [9]. Since 2004 we have verified all new isolates as well as 32 isolates from earlier years that were stored on stock agar by the Vitek 1 identification and susceptibility system (bioMérieux Ltd, UK).
Pulsed-field gel electrophoresis (PFGE) analysis
The genetic relatedness of the S. sonnei isolates was examined by PFGE. Analysis was performed on 26 non-repeat isolates from MHMC patients in the years 2000–2007; 19 of the isolates were from residents of BB and seven from those of four other towns of Central Israel. The 19 BB isolates were selected according to the home address of the patients to represent different neighbourhoods of the city. Bacterial DNA was prepared and cleaved with 20 U XbaI endonuclease (New England Biolabs, USA) [Reference Brian10]. Plugs were loaded onto a 1% agarose gel (BMA Products, USA) prepared and run in 0·5 m Tris–borate-EDTA buffer on a CHEF-DR III apparatus (Bio-Rad Laboratories Inc., USA). Electrophoresis was performed at 6 V/cm for 23 h at 14°C. The pulse time increased from 5 s to 35 s. Gels were stained with ethidium bromide, washed in distilled water, and photographed in UV light using a Bio-Rad GelDoc camera (Bio-Rad). PFGE DNA macro-restriction patterns were visually compared and interpreted according to the criteria established by Tenover et al. [Reference Tenover11].
Statistical analysis
Disease incidence is presented as the number of cases per month during the years 1998–2006. Crude incidence rates were calculated by the number of cases divided by the corresponding population (retrieved from the annual publications of the Israeli Bureau of Statistics, available at: www.cbs.gov.il). Rates were calculated for BB, the Tel Aviv District, and the cities of Tel Aviv district other than BB (number of cases and population calculated as: district – BB). Age-specific incidence rates were calculated for the years 2000–2006 for two age groups: children aged <5 years, and those aged ⩾5 years. These rates were based on number of cases reported to the Ministry of Health at each age stratum.
Mann–Whitney test was used to assess the before-and-after effect comparing the distribution of cases per month between January 1998 and March 2002 (the last month before the beginning of the first intervention) with the monthly cases in the time between April 2002 and December 2006. These analyses were conducted using the SPSS statistical package for Windows, version 15.0 (SPSS Inc., USA).
Within-year seasonality was estimated by using Freedman's test for deviation from a uniform incidence [Reference Freedman12]. Two-month peaks were estimated by Ratchet circular scan test for a short seasonal peak [Reference Wallenstein, Weinberg and Gould13]. These analyses were performed with the WINPEPI statistical package [Reference Abramson14].
RESULTS
Surveillance data
During the years 1998–2006, 8450 cases of laboratory-confirmed shigellosis were reported to the Tel Aviv Health District Authority, 2122 (25·1%) of which were residents of BB. The annual crude rates in BB ranged between 18 (2003) and 353 (1998) per 100 000 population (Table 1). BB incurred higher incidence rates of shigellosis than other cities of the Tel Aviv district combined (Fig. 1).
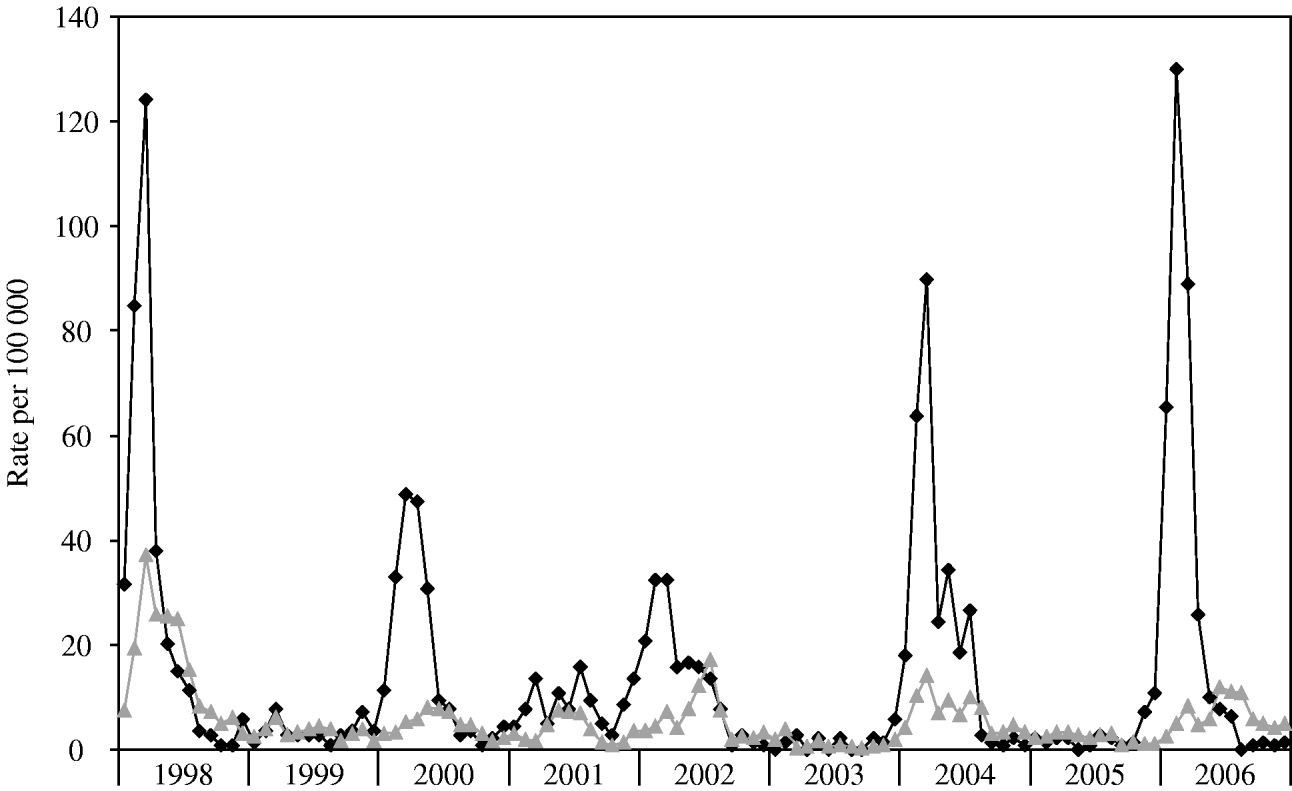
Fig. 1. Incidence rates per 100 000 population by month, 1998–2006, for Bene Beraq (–◆–) and other (![]() ) cities in the Tel Aviv district.
) cities in the Tel Aviv district.
Table 1. Shigellosis: incidence rates and rate ratios by year, Bene Beraq, 1998–2006
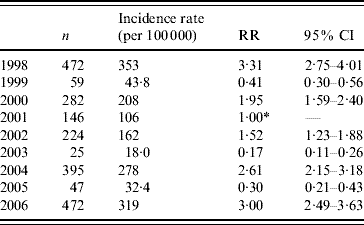
RR, Relative risk; CI, confidence interval.
* Reference category=2001.
In BB, patients in the 0–4 years age group accounted for about 60% of cases every year (range 57–87%). There were no gender differences between cases. The annual age-specific incidence rates per 100 000 in the 0–4 years age group ranged between 91 in 2003 and 1613 in 2006. These rates were 8–41 times higher than those for all other age groups combined (Table 2).
Table 2. Shigellosis: age-specific incidence rates per 100 000 and rate ratios of children aged 0–4 vs. 5 years, by year, Bene Beraq, 2000–2006
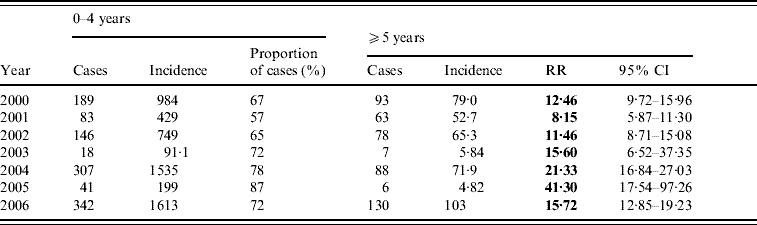
RR, Relative risk; CI, confidence interval.
Pattern of outbreaks by year
Outbreaks occurred in 1998, 2000, 2002, 2004, and 2006 (Fig. 1). This biennial pattern is supported by the annual incidence rate ratios of each year compared with 2001 (Table 1). Outbreaks in BB started before and exceeded in magnitude the outbreaks in the rest of the district (Fig. 1).
Within-year seasonality
Combining data from all nine years, there was a clear overall wintertime seasonality in BB, with 2-month peaks in February–March (P<0·005). Annual data showed clear seasonality in 1998, 2000, 2002, 2004, and 2006.
In all other cities of Tel Aviv district combined, the overall seasonality for the 9 years showed a summertime peak in June–July. Summer peak incidence was evident in 2000, 2001, 2002, and 2006 (May–June for 2000, and June–July for 2001, 2002, and 2006, P for every year <0·005). In 1999, 2003 and 2004 illness peaked in February–March (P<0·005), and in 1998 and 2005 incidence peaked in March–April (P<0·005).
MHMC data
Between 21 February 2000 and 31 December 2006, 6357 stool specimens were obtained from patients with diarrhoea who were referred to MHMC. Of these, 421 non-repetitive cultures were positive for S. sonnei (6·6%), 13 for S. flexneri (0·2%) and one for S. boydii (0·02%).
The pattern of illness as reflected in Shigella-positive cultures from MHMC is illustrated in Figure 2. Despite the fact that about half the patients admitted to MHMC were not residents of BB, trends in shigellosis in MHMC resembled those seen for the city of BB.
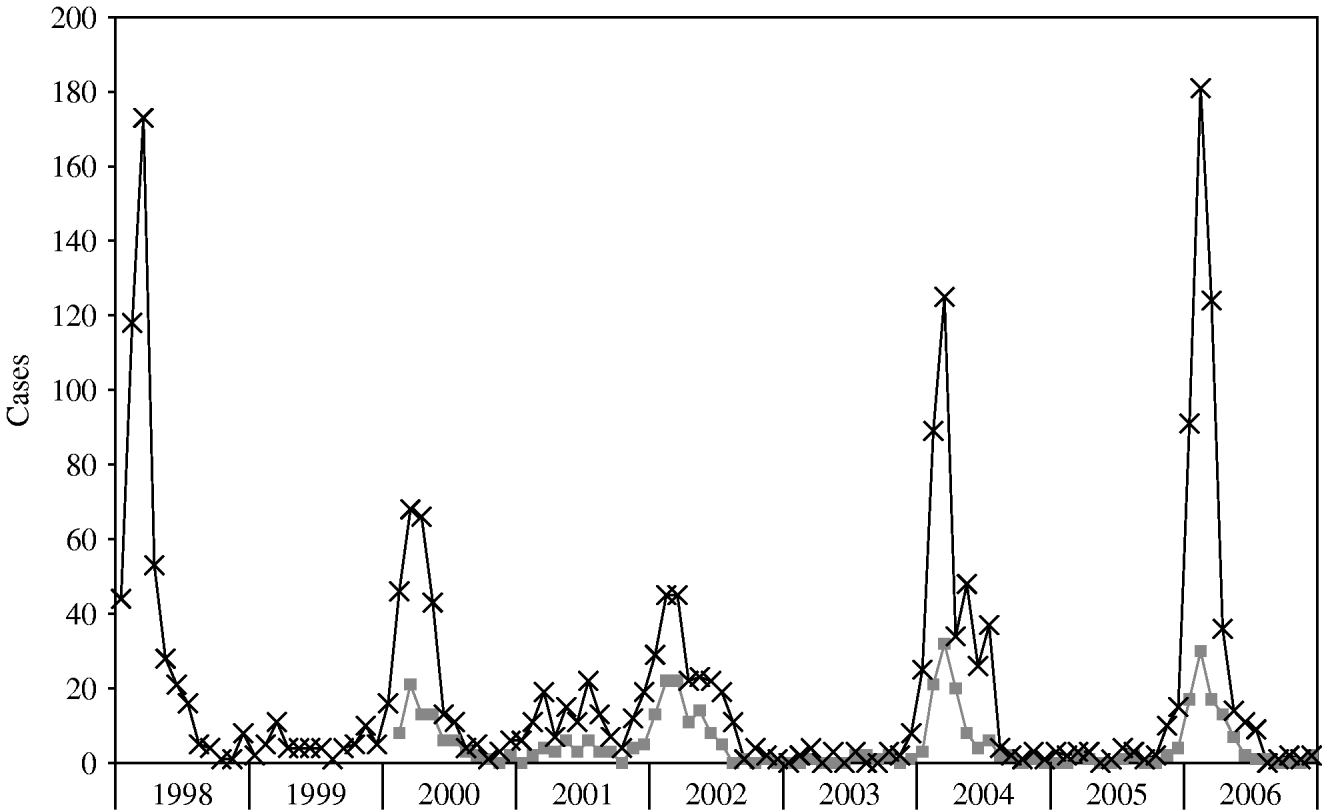
Fig. 2. Shigellosis cases by month, 1998–2006. –×–, Bene Beraq reports to Ministry of Health; ![]() , MHMC positive stool cultures. (MHMC isolates obtained from Ma'aynei Hayeshua Medical Centre, Bene Beraq, data obtained from the Ministry of Health.)
, MHMC positive stool cultures. (MHMC isolates obtained from Ma'aynei Hayeshua Medical Centre, Bene Beraq, data obtained from the Ministry of Health.)
Of patients with laboratory-confirmed shigellosis who were admitted to MHMC, the median age on admission was 4 years (range 0–95). Most (55%) were female, with no significant differences in gender distribution throughout the study period. The age distribution was different with a median of 3 years for males and 4 years for females. Of those aged ⩾16 years, 17% were female, compared with 5% who were male (P=0·03, Mann–Whitney test for age distribution).
Bacteriology: S. sonnei
Of the 421 MHMC isolates, all reacted typically for S. sonnei in biochemical tests and in all cases tested, the identification was confirmed by the Vitek1 system. All but one of the isolates proved rhamnose negative.
All isolates were susceptible to gentamicin, quinolones, and to all cephalosporins tested (including first-generation cephalosporins). More than 95% of the isolates were resistant to sulfamethoxazole–trimethoprim, and all but two isolates were resistant to ampicillin (AMP); these two isolates showed a distinct PFGE pattern (pattern B, see Table 3). Isolates tested by Vitek proved resistant to piperacillin (MIC ⩾256 mg/ml) but sensitive to piperacillin/tazobactam (<8 mg/ml). For AMP/clavulanic acid (AMC) and AMP/sulbactam, most isolates tested by Vitek were reported to be resistant (MIC ⩾32 mg/ml) and a few as intermediate. By disk diffusion, the isolates were either intermediate or resistant to AMC.
Table 3. PFGE patterns of 26 S. sonnei isolates from MHMC, 2000–2007
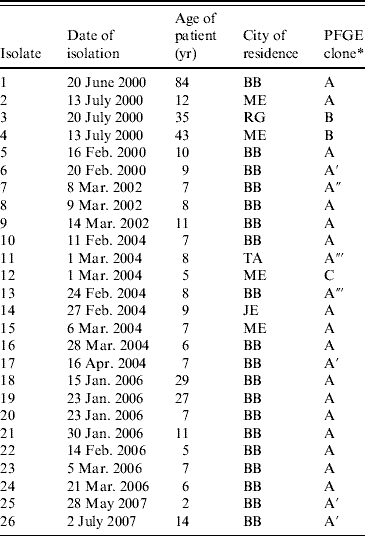
MHMC. Ma'aynei Hayeshua Medical Centre; BB, Bene Beraq; ME, Modiin Elith; TA, Tel Aviv; RG, Ramat Gan; JE, Jerusalem.
* Clones designated with the same letter showed the same PFGE restriction pattern. Clones A′, A″ and A‴ were epidemiologically related to clone A, but differed from clone A in one, two or three restriction bands, respectively.
PFGE
Of the 26 isolates of S. sonnei obtained in MHMC, 23 exhibited closely related XbaI restriction fragment patterns demonstrating a dominant outbreak clone (designated A). This clone occurred throughout the years from 2000 to 2006 (Table 3) and occurred in patients from four different cities. Of the three isolates that were not of pattern A, two (pattern B) were in 2000 and as mentioned above they were also distinctive in being susceptible to AMP.
Assessment of interventions
Following the first intervention that was initiated in April 2002, a decline in incidence was seen, and only two patients were admitted to MHMC with shigellosis between September and December 2002. Similarly, following the interventions in March 2004 and 2006, declines in incidence were seen (for a more detailed graph of 2000–2004, see Fig. 3).
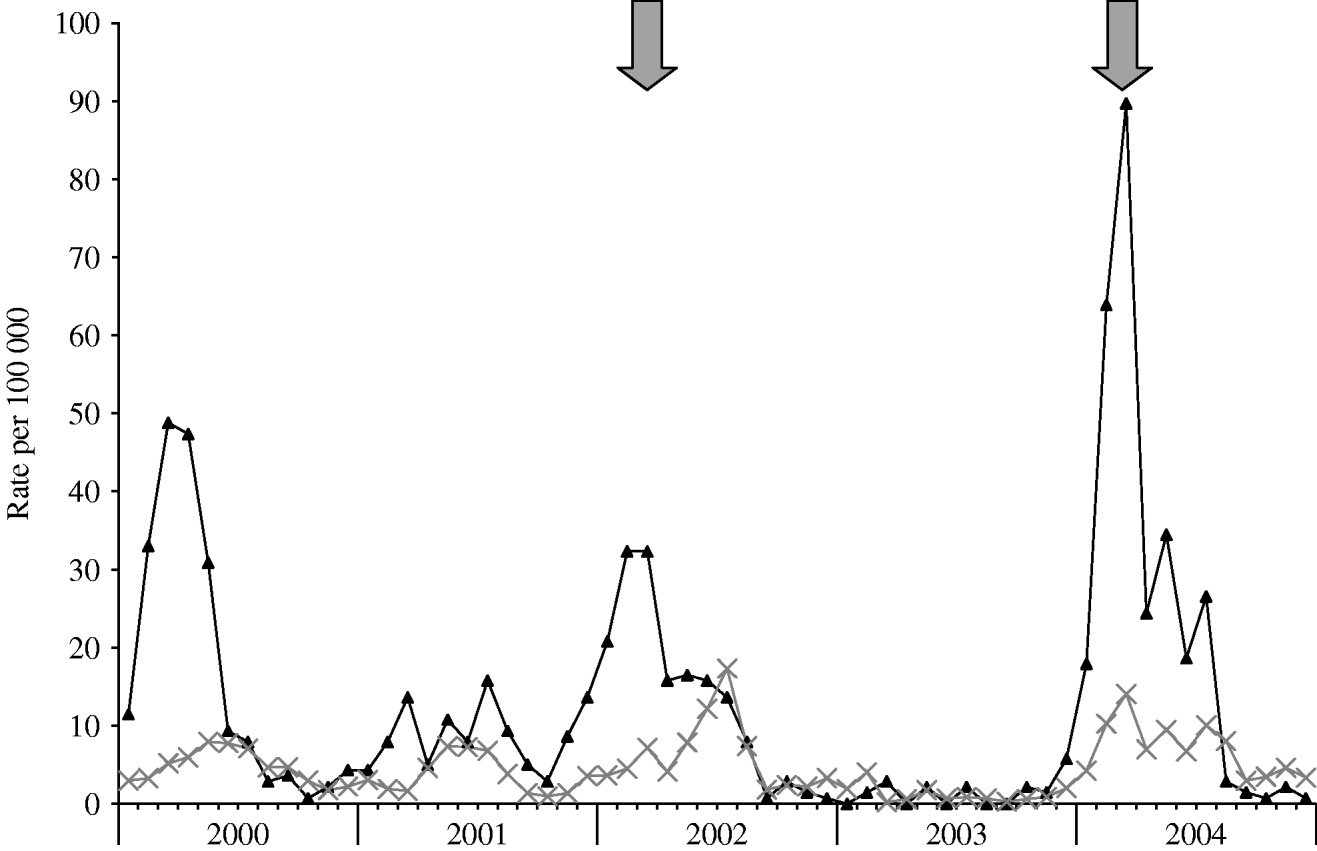
Fig. 3. Rates of shigellosis in 2002–2004 for Bene Beraq (–▴–) and other cities (![]() ) of the Tel Aviv district. Arrows indicate beginning of interventions.
) of the Tel Aviv district. Arrows indicate beginning of interventions.
Comparing the distribution of disease before and after the intervention of 2002 showed a significant decline in incidence after the interventions (Mann–Whitney, P=0·003), despite a continued pattern of biennial outbreaks and seemingly higher incidence rates during the outbreaks of 2004 and 2006. This also held true when the epidemic months (January–April of every outbreak year) were excluded from the analysis (Mann–Whitney, P=0·004). For the whole district, there was a borderline before-and-after association for the same periods (P=0·044); however, by excluding BB from this analysis cities in the Tel Aviv district had no significant reduction in incidence (P=0·091) after the BB interventions.
DISCUSSION
Our study demonstrated high incidence rates of shigellosis and a biennial pattern of outbreaks in wintertime in an observant religious community in Israel.
During the study period, the population of BB developed shigellosis with incidence rates that were similar to those reported in developing countries [Reference Ram15], and about ten times higher than those reported for the USA [Reference Gupta2, Reference Shiferaw16]. Similarly, the isolation rate (6%) of shigellosis in MHMC in all patients with diarrhoea was similar to those reported in developing countries in Asia and Africa [Reference Ram15]. However, unlike developing countries, and in common with developed countries, the predominant strain in BB was S. sonnei. Outbreaks in BB were caused by clonally related strains of S. sonnei, as suggested by the PFGE test, the antibiotic resistance pattern and the rhamnose-negative trait [17]. The pattern of antibiotic resistance in the current study was similar to that reported in another medical centre in a neighbouring city in Israel [Reference Ashkenazi18], with the exception of AMC. The resistance pattern to β-lactam drugs and β-lactam–lactamase combinations found in our isolates is rather uncommon in Enterobacteriaceae [Reference Dubois19]. The molecular basis for this particular resistance pattern will be investigated in a future study; however, its consistency across the years sustains the hypothesis that outbreaks were caused by genetically related strains.
Outbreaks occurred in 1998, 2000, 2002, 2004, and 2006. A recent report from Jerusalem [Reference Stein-Zamir20] shows shigellosis outbreaks in these same years. This could be attributed to the high proportion of observant Jews in the population of Jerusalem, and to the close contacts between these communities, as was shown in non-BB residents that were admitted to MHMC during the study period. It was similar to the previously demonstrated simultaneous outbreaks in distant observant communities in the USA [Reference Sobel5]. As would be expected from this biennial pattern, an outbreak occurred again in the winter of 2008 (data not shown). As serotype-specific immunity has been shown to develop after infections with Shigella [Reference Ferreccio21], we hypothesize that the biennial pattern might be due to the clonally related causative agents, and to the developed immunity in infected children, requiring about 2 years to allow the introduction of a sufficiently large number of ‘new’ susceptible children to day-care settings to cause an outbreak.
Outbreaks in BB occurred in wintertime. This stands in contrast to reports from other countries where the peak season was summer [Reference Wang22–Reference Naumova24]. In England, wintertime seasonality of shigellosis was reported in the 1950s [Reference Christie25], but more recent reports do not show distinct seasonality [Reference Newman26]. Other reports from Israel show outbreaks both in summer and winter [Reference Ferreccio21, Reference Finkelman27, Reference Ostroi, Anis and Green28]. As opposed to summertime, outbreaks in winter correspond with day-care attendance and with long hours of indoor activities associated with increased classroom density. Outbreaks peaked in BB earlier than the rest of the Tel Aviv district. This might suggest a propagation of disease from BB to residents of the nearby towns and cities, despite the relative social segregation of its population. Our study also suggests that endemic shigellosis in BB poses a health threat to surrounding communities.
During the three large outbreaks of 2002, 2004, and 2006, interventions were conducted at the community level and were based on educational efforts to improve personal hygiene and keep infected children at home. Methods included educational conferences for caregivers, public releases and the local media. As claimed by Mohle-Boetani et al. [Reference Mohle-Boetani29] we assumed that an effective intervention should use public releases. Private day-care institutions might fail to comply with hygiene requirements, especially those for keeping ill children at home. However once their ‘customers’ (i.e. parents) are convinced by public releases that infections are preventable, they would demand compliance from the caregivers. Indeed, after every intervention the epidemiological nurse received many responses from caregivers, with some parents informing her how the sanitation had been improved in their facilities, and that only the publicity highlighting this problem had enabled the caregivers to maintain the recommendation of keeping sick children at home. Unfortunately, these responses were not quantified.
It is difficult to decide regarding the short-term effectiveness of our interventions, whether they did indeed affect the natural course of the outbreaks. Following the intervention in 2002, very few cases of shigellosis occurred for a year and a half, compared with the inter-outbreak periods following the outbreaks of 1998 and 2000. While local interventions may have reduced illness, they were not effective in preventing the next outbreaks. The value of personal hygiene in preventing shigellosis is well established [Reference Curtis and Cairncross30–32]; however, we were unable to find studies on the effectiveness of interventions in terminating a non-common-source outbreak in a large community and the frequency needed to maintain a long lasting high level of compliance.
Our study has some limitations – surveillance data might be biased over the years by other active surveys, by physicians' awareness regarding outbreaks and pathogens, and by their practice of referring patients for stool culture and reporting to the Ministry of Health. It is reasonable to conclude that reported cases are only the tip of the iceberg in terms of the real scope of disease [Reference Doyle, Glynn and Groselclose33], but we have no reason to suspect that the biennial pattern shown here does not represent the pattern of outbreaks in this community. Other limitations include lack of clinical and follow-up data on patients. The strength of this study is in the long-term surveillance of a unique population.
MHMC is a small community-based hospital without financial resources for outreach programmes in the community. Despite this constraint, this study showed the importance of a community hospital as a sentinel laboratory that can detect outbreaks on a real-time basis, and the ability of such a small hospital to serve as the motivating force for an intervention of health promotion in the community. Future studies are needed to assess the most effective, community-based, ongoing interventions to be implemented in susceptible populations living in developed countries with clean water supply. An intriguing question is whether an intervention implemented in BB before an expected biennial recurrence (e.g. in November–December 2009) could prevent an outbreak.
ACKNOWLEDGEMENTS
The authors acknowledge Dr R. Schneerson, Dr J. B. Robbins from the Fogarty International Center, National Institute of Health, for their useful comments, Rabbi Jonathan Safra, Head of the Department of Education, Municipality of Bene Beraq, for his collaboration, and Dr C. Mintzer-Ophir from the Pediatrics Department, MHMC, for her assistance in conducting this study.
DECLARATION OF INTEREST
None.










