INTRODUCTION
Salmonellosis is a gastrointestinal infection largely caused by non-typhoidal serovars of Salmonella bacteria. Non-typhoidal salmonellosis occurs worldwide and it is estimated that there are 93·8 million cases per year, resulting in about 155 000 deaths [Reference Majowicz1]. Salmonella enterica subsp. enterica serovar Mikawasima (S. Mikawasima) is a rare, non-typhoidal serovar of Salmonella. There is limited information on this serovar in the literature, although previous studies have found S. Mikawasima present in environmental water samples [Reference Polo2]. Additionally, one outbreak that occurred in the UK in October 1992 identified an association between S. Mikawasima infection and eating doner kebabs from a takeaway food outlet [Reference Synnott3].
Proton pump inhibitors (PPIs) are widely used in the treatment of gastroesophageal diseases and are available over the counter without prescription in many countries. They work by inhibiting H+/K+-ATPase activity in the stomach, resulting in the suppression of acid secretion and thus reducing stomach acidity [Reference Sachs, Shin and Howden4]. Several case-control, cohort and ecological studies have identified associations between PPI usage and an increased risk of gastroenteritis caused by bacterial pathogens, including Campylobacter spp. [Reference Neal5–Reference Bouwknegt7] and Clostridium difficile [Reference Dial8, Reference Jayatilaka9]. A 2006 case-control study conducted by Doorduyn and colleagues found that PPI usage was associated with increased risk of Salmonella Enteritidis and Typhimurium infection [Reference Doorduyn10]. However, there is debate around whether PPI usage increases the risk of developing salmonellosis, with authors of a large cohort study concluding that there was no evidence to suggest that PPIs were associated Campylobacter or Salmonella infection since patients prescribed PPIs have greater underlying risk for gastrointestinal infection [Reference Brophy11].
In October 2013 an increase in Salmonella spp. reports was noted in North East England. This was later identified as part of a national increase in cases of S. Mikawasima. Once an outbreak had been noted from reference laboratory data through automated exceedance reporting in England and Wales [Reference Farrington12] and routine monitoring of laboratory data in Scotland, a national multi-disciplinary outbreak control team was convened to investigate the situation. Initial investigations identified that a large proportion of cases reported taking PPIs prior to illness, although no clear exposure associated with illness was identified at this time from the hypothesis-generating questionnaires. We therefore decided that an analytical study should be conducted to investigate the outbreak with the purpose of fulfilling two main objectives: first, to identify exposure(s) associated with S. Mikawasima infection and; second, to investigate if any association between PPI usage and S. Mikawasima infection existed.
METHODS
Microbiological
Isolates were referred to the Salmonella Reference Service, Public Health England (PHE) or the Scottish Salmonella, Shigella and Clostridium difficile Reference Laboratory for confirmation and typing. Serotyping to identify S. Mikawasima strains was carried out according to the White–Kauffmann–Le Minor classification [Reference Grimont and Weill13].
Epidemiological
We conducted a frequency-matched case-control study to test the hypotheses generated from the hypothesis-generating questionnaires. A case was defined as a person with gastroenteritis and a positive S. Mikawasima faecal specimen taken between 1 October 2013 and 29 November 2013. As specimen date was not available for the cases in Scotland, date of onset was used. Cases in Scotland were therefore included if they had a positive S. Mikawasima specimen and an onset of illness between 1 October 2013 and the 29 November 2013. Cases eligible for inclusion in the study were aged between 18 and 65 years (inclusive) and were residents in England, Wales or Scotland. We excluded cases from the study if they reported travel outside of Europe (including Turkey) or being in contact with another person with diarrhoeal symptoms in the 7 days before their symptom onset.
PHE Centre staff members (or equivalent for Scotland) aged between 18 and 65 years were randomly selected to act as controls; two controls were recruited for each case. Controls were frequency-matched to cases by sex and reporting region. Regional centres are distributed across England and Scotland. Controls were excluded if they reported travel outside of Europe (including Turkey) or being in contact with another person with diarrhoeal symptoms in the 7 days before interview. Controls were also excluded if they reported having two or more of the following symptoms in the 7 days before interview: diarrhoea, blood in stool, nausea, vomiting, abdominal pain or fever. Cases and controls were interviewed via telephone and responses to questions were recorded on paper questionnaires.
In addition to questions on PPI usage in the 7 days before illness (or interview for controls), a range of food and environmental exposures were included in the questionnaire. We investigated exposure in the 3 days prior to illness for cases and 3 days prior to interview for controls for the following: consumption of fruit or salad vegetables, consumption or handling of poultry, consumption of herbs and spices, consumption of food outside the home (e.g. at restaurants), contact with wild birds and, outdoor walking activities. We also asked if any raw chicken (whole or portions) had been purchased in the 14 days prior to illness/interview, and if so, asked where the chicken was purchased (e.g. supermarket, local butcher). Due to the seasonal nature of S. Mikawasima cases observed in previous years, we asked if cases or controls had eaten or handled pumpkins or chestnuts in the 3 days prior to illness/interview. We entered questionnaire responses in duplicate into an EpiData database and data validation was performed. Where discrepancies occurred, the original questionnaire was consulted to provide the definitive response. If the response given in the questionnaire was still unclear, an independent data reviewer was consulted to decide which response should be recorded.
Cases were described by time, place and person. Odds ratios (ORs), 95% confidence intervals (CIs) and P values were calculated for exposure variables assessed in the study questionnaire. Exposures that had an estimated OR > 1 and P < 0·2 were included in a multivariable logistic regression model, where adjusted odds ratios (aORs) were estimated. Logistic regression analysis included age and sex to allow for any potential confounding these may have caused. Likelihood ratio tests were performed to assess whether an exposure variable remained associated with being a case, allowing for the other exposure and potential confounding variables in the regression model.
Data cleaning, manipulation and analysis were performed in Stata/SE version 12 (StataCorp., USA).
RESULTS
A total of 77 cases were reported up to 6 December 2013, when the case-control study commenced. The spatial distribution of cases in England and Wales reported prior to the study commencing is shown in Figure 1. Sixty-one cases were eligible for inclusion in the study based on the case definition described above. We received 40 case (response rate 65·6%) and 86 control questionnaires. One case had to be excluded because they had been in close contact with somebody with diarrhoea in the previous 7 days. Four control questionnaires were excluded: two controls had exhibited more than two clinical symptoms in the previous 7 days; one control had been in close contact with somebody with diarrhoea and; one control had travelled outside of Europe. A total of 39 laboratory-confirmed S. Mikawasima cases and 82 controls were therefore included in the analysis.

Fig. 1. Distribution of cases across England and Wales, October–November 2013 (postcode data for cases in Scotland was not available).
Specimen dates relating to cases included in the study ranged from 5 October 2013 to 22 November 2013. An epidemic curve of cases included in the study is shown in Figure 2. The median age for cases was 45 years (interquartile range 28–56) and 22 (56·4%) of cases were female. Table 1 summarizes the case and control characteristics in terms of age, sex, ethnicity and travel history. There was no evidence to suggest that cases differed from controls in terms of demography or travel history.

Fig. 2. Epidemic curve of S. Mikawasima cases included in the case-control study, October–November 2013.
Table 1. Summary of case and control characteristics
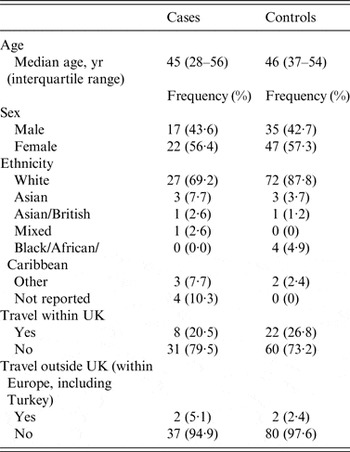
Table 2 lists the distribution of clinical symptoms. Other symptoms reported included tiredness, dehydration and flu-like symptoms. Thirty-three percent of cases were admitted to hospital as a result of their illness. Taking dietary supplements was reported by 10·3% (n = 4) of cases in the 7 days prior to illness, while 30·8% (n = 12) reported taking PPIs and 46·2% (n = 18) reported having an underlying health condition.
Table 2. Distribution of clinical symptoms reported by cases

Results from the univariable analysis indicated that PPI usage was associated with infection (Table 3). A high proportion of cases reported eating food outside of the home (82·1%). Several of the restaurant types and fast-food outlets met the criteria for entry into the multivariable analysis and therefore these exposures were analysed as grouped variables (Tables 3 and 4). As a large proportion of the cases and controls had not bought any chicken within 14 days of illness or interview (35/121, 28·9%), the non-supermarket chicken variable was split into its univariable components, with each analysed individually. After adjusting for age and sex, the results of the multivariable analysis indicated that infection was associated with PPI usage, eating chicken at restaurants, eating from fast-food outlets, as well as eating chicken from fast-food outlets, and purchasing chicken from local butchers. The results of the final multivariable model are given in Table 4.
Table 3. Summary of exposures associated with illness from the univariable analysis

OR, Odds ratio.
Table shows only variables eligible for entry into the multivariable model
Table 4. Results from the multivariable regression analysis

aOR, Adjusted odds ratio; CI, confidence interval.
DISCUSSION
Our investigation of a national outbreak has identified an independent association between S. Mikawasima infection and PPI usage in the 7 days prior to illness. Investigation into food and environmental exposures indicated that cases were more likely than controls to eat chicken from restaurants or fast-food outlets, or purchase chicken from local butchers. Eating at fast-food outlets, but not eating a chicken dish, was also associated with infection.
In the UK, PPIs are one of the most commonly prescribed groups of drugs and only low-dose (10 mg) omeprazole is available over the counter. Side-effects associated with taking a PPI include diarrhoea and vomiting, hypomagnesaemia and an increased risk of hip, wrist and spine fracture [14]. Guidelines have been published on PPI usage [15, 16]; however, a study by Batuwitage et al. found that they were often prescribed inappropriately [Reference Batuwitage17].
The role of PPIs in the development of gastroenteritis has been well documented for some gastrointestinal pathogens but their role in the pathogenesis of salmonellosis is unclear. A large cohort study utilizing routinely collected data reported that there was no association between PPI usage and infection caused by Salmonella or Campylobacter [Reference Brophy11]. The authors of this study found that patients prescribed PPIs had a greater risk of developing gastrointestinal infection prior to PPI prescription. These authors describe how patients taking PPI were more likely to have other factors associated with symptoms of gastroenteritis, such as antibiotic use, oral steroid use, non-steroidal anti-inflammatory drug prescription, bowel surgery, or a diagnosis of arthritis. Based on the findings from their analysis, the authors concluded that there was no evidence to suggest that PPI usage was associated with increased rates of gastrointestinal infection.
By contrast, a case-control study by Wu et al. found that recent PPI usage was associated with an increased risk of non-typhoidal salmonellosis [Reference Wu18]. The study matched for age, Charlson comorbidity index score and predisposing factors for non-typhoidal salmonellosis, such as gastrointestinal surgery, autoimmune diseases, transplantation and other malignancies. After adjusting for these factors, Wu et al. still found that patients with non-typhoidal salmonellosis were more likely to have used PPIs in the 12 months prior to infection (aOR 2·09, 95% CI 1·95–2·24). The strongest association was found in those taking PPIs at the time of non-typhoidal salmonellosis diagnosis (aOR 5·39, 95% CI 4·79–6·06).
A systematic review by Bavishi & Dupont concluded that gastric acid suppression resulting from PPI usage was associated with increased susceptibility to gastrointestinal infection caused by Salmonella, Campylobacter and C. difficile [Reference Bavishi and Dupont19]. Based on their findings, the authors of the review recommended that patients on PPIs should be informed that they are more susceptible to gastrointestinal illness caused by bacterial pathogens and should therefore be given appropriate dietary advice. Furthermore, an outbreak investigation performed by Bowen et al. identified an association between Salmonella Enteritidis infection and PPI usage [Reference Bowen20]. As a result of their investigation into the outbreak these authors recommended that risks and benefits should be assessed before acid-suppressing medication is prescribed to patients at high risk of developing complications following enteric infections. The findings from these studies, in addition to the present study, suggest that patients prescribed PPIs should be made aware of the risk of enteric infections and should seek medical advice if they develop symptoms.
Utilizing staff members as controls in the analytical study did result in rapid recruitment of a control population compared to other methods, such as sequential or random digit dialling. We recognize that there may be differences between the control population and the population from which the outbreak cases arose, particularly around comorbidities and occupational status. One limitation of our study was that by using staff members as controls, we did not ask questions about underlying health conditions and were therefore unable to compare comorbidities between cases and controls. As we may wish to investigate underlying medical conditions in future outbreak investigations, we will need to consider other rapid methods for recruiting controls [Reference Mook21], or getting consent from staff members to provide answers to health-related questions. Furthermore, it is possible that selecting staff members as controls may have resulted in an overestimate of the association between PPIs and salmonellosis in this study. Staff controls are likely to be a healthier than the population that cases arose from and therefore less likely to be taking medications such as PPIs. PPI usage in our controls was 4·9%. Parkin et al. found that 6–8% of females and 7–10% of males aged <65 years had one or more PPI prescription in the UK between 2004–2006 [Reference Parkin, Hagberg and Jick22]. These proportions are slightly higher than the proportion of PPI usage we found in our controls. However, the proportion of PPI usage in our controls is similar to PPI usage in other case-control studies investigating risk factors for gastroenteritis where controls were selected from the general population in England [Reference Tam6] and higher than PPI usage in general population control groups reported by other European countries [Reference Garcia Rodriguez, Ruigomez and Panes23, Reference Doorduyn24]. Therefore, there seems to be limited potential for over-estimation of the association between PPI and salmonellosis in our study.
A further limitation is that we restricted cases for our study to those aged 18–65 years. A recent study by Bouwknegt et al. identified an association between PPI prescriptions and campylobacteriosis [Reference Bouwknegt7]. They found that the effect of PPI prescription was largest for younger age groups and decreased in older age groups. This effect may also exist for salmonellosis and we therefore recommend that further studies investigating associations between PPIs and salmonellosis include older age groups.
Some cases eligible for inclusion in the case-control study were not included in the analysis (34·4%) as, due to limited resources, some regions were not able to follow-up a proportion of cases in their area. Although this reduced the power of the analytical study, re-performing the power calculations using the final number of cases and controls available indicated that the study was still able to detect moderate increases in OR for common exposures.
Unfortunately, it was not possible to trace the chicken back to the supplier. This was due to the variety of venues reported by cases and wide geographical distribution of cases across the UK. Since there was no obvious vehicle of infection identified from the trawling questionnaires, there was no way of rapidly identifying and focusing on a potential source of infection. Results of this outbreak investigation can be used to aid in the rapid investigation of any future S. Mikawasima outbreaks, which may allow the traceback of suspected chicken products to the supplier.
Salmonella spp. are acid-sensitive bacteria [Reference Karatzas25, Reference Riesenberg-Wilmes26]. PPI are known to alter the pH and microflora of the upper gastrointestinal tract, which can facilitate the multiplication of pathogens. Specifically, PPI interact with the tight junctions of intestinal cells, promoting adhesion of Salmonella which may result in increased pathogenicity [Reference Bavishi and Dupont19]. Further microbiological studies are required to test this hypothesis and provide us with a clearer understanding of the role of PPIs in the pathogenesis of salmonellosis. In addition, more evidence is required on host susceptibility and salmonellosis, and outbreak investigation provides a good opportunity to investigate these factors further. We have identified an association between PPI usage and infection with S. Mikawasima through conducting an analytical study as part of our outbreak response. We recommend that future outbreak investigations should include questions on PPI usage and underlying health conditions to strengthen the evidence on the relevance of PPIs in Salmonella infection. The information generated from such studies can then be used to further develop guidelines on PPI usage and help inform prescribers and patients of the risks associated with PPI usage.
ACKNOWLEDGEMENTS
The authors acknowledge public health, microbiology and food safety colleagues in England, Wales and Scotland who assisted with this outbreak investigation.
This investigation was part of the routine public health functions of the participating organizations and so no additional funding was sought.
DECLARATION OF INTEREST
None.









