INTRODUCTION
The Chinese government has taken many measures to deal with its long-term tuberculosis (TB) burden and great success has been achieved in terms of increased case detection and treatment success in the past decades [Reference Chen1–Reference Wang, Liu and Chine3]. But against this backdrop, efforts for TB control have been challenged by the emergence and wide spread of drug-resistant TB cases, especially multidrug-resistant (MDR)-TB and extensively drug-resistant (XDR)-TB cases in recent years [Reference Wright4]. According to the fourth report on global anti-tuberculosis drug resistance, the global estimated number of incident MDR-TB cases in 2006 was 489 139, and China ranks first with an estimated total number of 130 548 [5].
As drug resistance in Mycobacterium tuberculosis shows marked geographic variation, periodic assessment of the patterns and trends of drug-resistant TB could help to monitor the effects of TB control programmes and to make appropriate adjustments in the treatment strategy as resistance patterns change over time for different localities. However, limited information is available on the extent of drug resistance in TB cases, especially those seeking treatment in hospitals in China. We thus undertook this retrospective study to determine the susceptibility patterns and trends of drug resistance in TB patients in the 309 Hospital in Beijing, China for the period 1997–2009.
METHODS
Study subjects and data collection
The 309 Hospital is the only TB referral hospital in the urban area of Beijing, and it is also one of the major TB referral hospitals in China with a 244-bed TB centre providing diagnostic and therapeutic services for TB patients throughout the country. The patients treated at the 309 Hospital are either self-referred or referred by clinicians from the general hospitals, community clinics, as well as district TB prevention and treatment clinics in Beijing and other provinces in China. All pulmonary and extrapulmonary TB patients with positive culture results who were tested for drug resistance at the 309 Hospital from January 1997 to December 2009 were included in this study. The medical records were reviewed for age, gender, TB treatment history, and specimen sites. The study was approved by the Ethics Committee of the 309 Hospital.
Cultures and drug susceptibility test (DST)
Cultures were performed using the Bactec MGIT 960 system (Becton Dickinson Diagnostic Systems, USA) according to the manufacturer's instruction. M. tuberculosis isolates were identified by acid-fast staining of sputum smear and biochemical tests including the para-nitrobenzoic acid test and the thiophene-2-carboxylic acid hydrazide resistance test, and were further confirmed by PCR. Mycobacteria other than M. tuberculosis complex were excluded from the analysis.
DST was performed using the conventional proportion method with Löwenstein–Jensen medium. The concentrations of the drugs used were as follows: isoniazid (1 mg/l), rifampin (50 mg/l), ethambutol (5 mg/l), streptomycin (10 mg/l), ofloxacin (2 mg/l), levofloxacin (2 mg/l), kanamycin (10 mg/l), aminosalicylate (1 mg/l). Quality control was routinely performed during susceptibility testing using the reference strains provided by the National Institute for the Control of Pharmaceutical and Biological Products (China). Periodic external quality assessment of the performance of DST results was conducted by the Tuberculosis Reference Laboratory (TRL) at the Beijing Research Institute for Tuberculosis Control. All drugs were obtained from Sigma Life Science Company (USA).
Definitions
Mono-resistant TB was defined as resistance to a single first-line anti-TB drug. Poly-resistant TB was defined as resistance to two or more of the first-line anti-TB drugs, but not to both isoniazid and rifampin [6]. MDR-TB was defined as resistance to at least isoniazid and rifampin. XDR-TB was defined as resistance to isoniazid and rifampin, plus any fluoroquinolone and at least one of three injectable second-line drugs: amikacin, capreomycin or kanamycin [7, 8].
Statistical analysis
Statistical analysis was performed using SPSS version 15.0 statistical software (SPSS Inc., USA). Pearson's χ2 test method was used to compare categorical variables. Trends in drug resistance for all TB cases or those who were Beijing residents during the specified period were examined using χ2 linear trend analysis. A P value of <0·05 was considered statistically significant. In addition, we further compared trends in drug resistance for Beijing residents aged ⩽65 years and >65 years.
RESULTS
Characteristics of study subjects
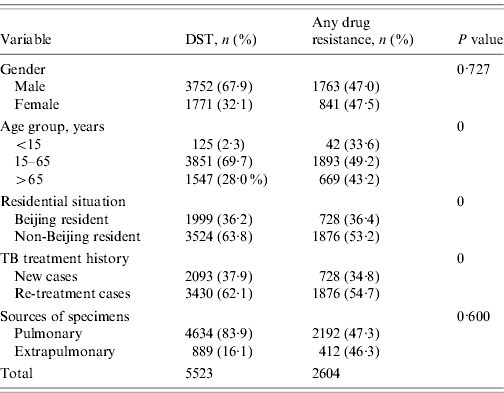
From January 1997 to December 2009, 5871 non-repetitive TB patients who had M. tuberculosis isolates were subjected to DST for all eight anti-TB drugs listed (see Methods section) at the 309 Hospital. There were 348 patients (5·9%) excluded as a result of non-viable specimens or contaminated cultures. Of the remaining 5523 patients, 3752 (67·9%) were male; 3430 (62·1%) were re-treatment cases; 1999 (36·2%) were Beijing residents and the rest were from other provinces of China (non-Beijing residents). The mean (±s.d.) age of the study subjects was 45·8±20·2 years (range 0·5–98·0 years). The patients were categorized into three age groups: <15 years (n=125, 2·3%), 15–65 years (n=3851, 69·7%), >65 years (n=1547, 28·0%). There were 4634 (83·9%) isolates obtained from pulmonary specimens and 889 (16·1%) isolates obtained from extrapulmonary sites (Table 1). Drug-resistant TB was significantly higher in patients aged 15–65 years (P<0·001), who were non-Beijing residents (P<0·001), and who were re-treatment cases (P<0·001). We did not observe significant differences in drug resistance in terms of the gender of the patients or the source of the specimens (Table 1).
Table 1. Characteristics of TB patients with drug susceptibility test (DST) results in the 309 Hospital, 1997–2009
Rates of resistance to anti-TB drugs in TB patients, 1997–2009
During 1997–2009, in the 5523 tested patients, 2604 had resistance to any anti-TB drug (47·1%). The resistance rates of TB patients to any single anti-TB drug were as follows: isoniazid, 1669 (30·2%); rifampin, 1571 (28·4%); ethambutol, 1455 (26·3%); streptomycin, 1477 (26·7%); kanamycin, 828 (15·0%); ofloxacin, 536 (9·7%); levofloxacin, 308 (5·6%); and para-amino salicylic acid, 912 (16·5%). The rates of TB patients with mono-resistant TB, poly-resistant TB, MDR-TB and XDR-TB were 14·8%, 19·8%, 19·4%, and 1·3%, respectively. More detailed information of drug resistance rates with regard to TB treatment history (new or re-treatment cases) are shown in Table 2.
Table 2. Anti-TB drug resistance profile of TB patients with drug susceptibility test results at the 309 Hospital, 1997–2009

H, Isoniazid; R, rifampicin; E, ethambutol; S, streptomycin; KM, kanamycin; OFX, ofloxacin; LVX, levofloxacin; PAS, para-amino salicylic acid; MDR, multidrug-resistant; XDR, extensively drug-resistant; TB, tuberculosis; DST, drug susceptibility test.
Trends in drug resistance in TB patients, 1997–2009
During 1997–2000, the percentage of patients with any resistance, mono-resistant TB, poly-resistant TB, MDR-TB and XDR-TB increased from 46·5%, 12·6%, 10·7%, 26·4%, and 1·9% in 1997 to 84·2%, 34·5%, 26·6%, 56·8%, and 7·9% in 2000, respectively (P<0·001 for all). During 2000–2003, while the percentage of patients with any resistance, mono-resistant TB, and poly-resistant TB continued to increase (although more slowly), the percentage of patients with MDR-TB and XDR-TB declined significantly from 56·8% and 7·9% in 2000 to 24·4% and 1·3% in 2003, respectively. In 2004, we observed a rebounding in the percentage of TB patients with different susceptibility patterns with the exception of mono-resistant TB. During 2004–2009, the percentage of patients with any resistance, mono-resistant TB, poly-resistant TB, MDR-TB and XDR-TB decreased significantly from 93·2%, 35·0%, 35·0%, 38·9%, and 2·5% in 2004 to 23·8%, 6·0%, 8·9%, 10·8% and 0·2% in 2009, respectively (P<0·001 for all) (Fig. 1).
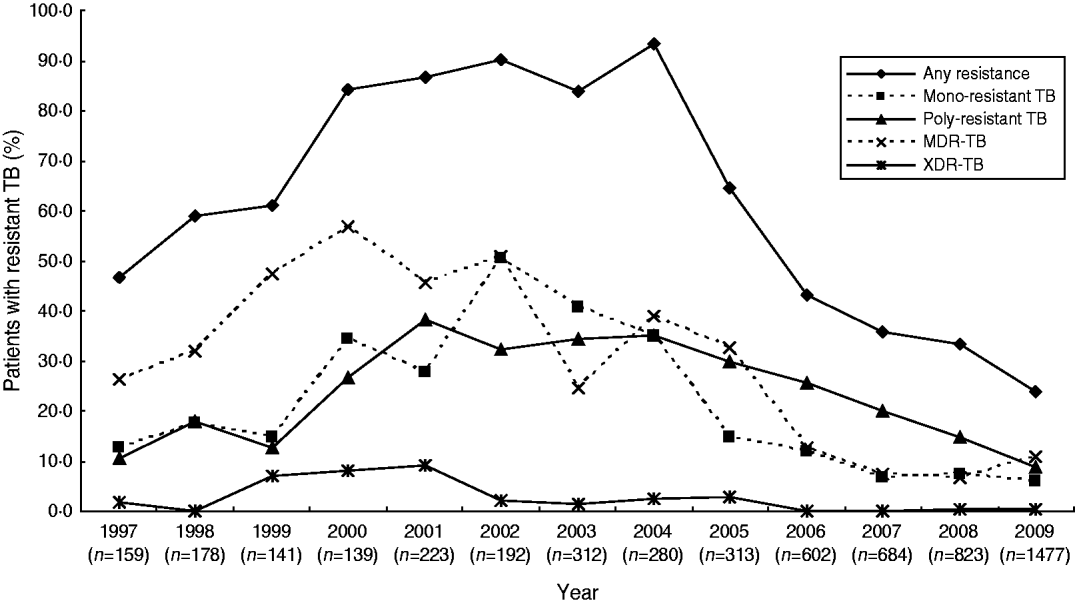
Fig. 1. Anti-tuberculosis drug resistance trends in TB cases at the 309 Hospital, 1997–2009.
Prevalence and trends of drug-resistant TB in patients who were Beijing residents, 1997–2009
Since the proportion of TB patients who were non-Beijing residents may have unpredictably fluctuated over the observation time, we performed analysis for patients who were Beijing residents only. The rates of patients with any resistance, mono-resistant TB, poly-resistant TB, MDR-TB and XDR-TB were 36·4%, 12·7%, 16·9%, 18·2%, and 1·4%, respectively. During 1997–2000, the percentage of patients with any resistance, mono-resistant TB, poly-resistant TB, MDR-TB and XDR-TB increased significantly from 38·4%, 12·3%, 13·7%, 17·8%, and 0% in 1997 to 54·5%, 21·5%, 24·1%, 25·3%, and 5·1% in 2000, respectively (P<0·001 for all). During 2000–2003, the percentage of patients with XDR-TB declined significantly from 5·1% in 2000 to 1·6% in 2003 (P<0·001), and the increasing trends of patients with other susceptibility patterns also began to be reversed during this period. In 2004, we observed a rebounding in the percentage of TB patients with MDR-TB and XDR-TB. During 2004–2009, the percentage of patients with any resistance, mono-resistant TB, poly-resistant TB, MDR-TB and XDR-TB decreased significantly from 47·7%, 18·2%, 22·7%, 25·0%, and 2·3% in 2004 to 22·5%, 6·2%, 10·5%, 11·1%, and 0·3% in 2009, respectively (P<0·001 for all) (Fig. 2).

Fig. 2. Anti-tuberculosis drug resistance trends in TB cases who were Beijing residents.
Prevalence and trends of drug-resistant TB in patients who were Beijing residents stratified by age, 1997–2009
Since age could be a key variable for the chances of TB patients having drug resistance, we further stratified TB patients who were Beijing residents into two age groups (⩽65 years and >65 years) and compared the prevalence and trends of drug-resistant TB for them. Of those aged ⩽65 years, the rates of patients with any resistance, mono-resistant TB, poly-resistant TB, MDR-TB and XDR-TB were 38·7%, 9·7%, 17·9%, 17·1%, and 1·2%, respectively. Of those aged >65 years, the rates of patients with any resistance, mono-resistant TB, poly-resistant TB, MDR-TB and XDR-TB were 31·3%, 19·3%, 14·6%, 20·6%, and 1·8%, respectively. In addition, we observed an overall lower percentage of mono-resistant TB, MDR-TB and XDR-TB, while observing a relatively higher percentage of any resistance and poly-resistant TB in younger patients (⩽65 years) than older patients (>65 years) over the years. More detailed information on trends of drug-resistant TB for the two age groups are shown in Figure 3(a, b).

Fig. 3. Anti-tuberculosis drug resistance trends in TB cases who were Beijing residents stratified by age. (a) Patients aged ⩽65 years; (b) patients aged >65 years.
DISCUSSION
Hospitals have played an important role in TB treatment in China, but the current situation with drug-resistant TB cases in hospitals has remained largely unknown [Reference Wang, Liu and Chine3, Reference Wang9]. Beijing, where TB control facilities were established as early as 1952 and where directly observed therapy, short-course (DOTS) strategy (recommended by the WHO) was started in 1978, has been one of the cities with the lowest TB burden in China [Reference Zhang10–Reference He12]. However, with an increasing urban migrant population and a large number of TB patients from other provinces seeking treatment in Beijing hospitals, a dangerous reservoir could be produced for transmission of drug-resistant TB [Reference Jia13, Reference Wei14]. Indeed, our hospital-based study documented much higher rates of drug resistant-TB cases compared to the results from a general population-based survey for Beijing city conducted by He et al. in which, of 1197 cases tested, the prevalence of patients with any resistance and MDR-TB were 20·1% and 3·5%, respectively, in Beijing for 2004 [Reference He12], while in our study, the rates of total resistance and MDR-TB in all TB patients were 93·2% and 38·9%, respectively, and the rates of total resistance and MDR-TB in Beijing residents were 47·7% and 25·0%, respectively, in 2004. This could be mainly explained by different settings and sampling methods used in the two studies. In He et al.'s survey [Reference He12], only newly registered TB patients from the Beijing area were selected, the majority of which were new cases (87·1%, 1043/1197), while in our study subjects were mainly recurrent TB cases (62·1%), which characteristic was shown to be associated with drug-resistant TB in our study and many other studies [Reference Ming15–Reference Djuretic18]. In addition, the majority of our TB cases (63·8%) were from other provinces of China, and TB control in most of the other provinces was shown to be much worse than that in Beijing [2, Reference Wright4], as was also observed in our study. The marked high prevalence of drug-resistant TB cases observed in our hospital for 2004 could also be partly explained by the severe acute respiratory syndrome (SARS) epidemic which broke out and spread during 2003–2004 and occupied the majority of attention and resources for respiratory infectious disease control in most hospitals for the whole country at that time. However, the rates of resistant-TB cases for 2007 (36·0%, 7·2%, and 0% for any resistance, MDR-TB and XDR-TB, respectively) and 2008 (33·4%, 6·8%, and 0·2% for any resistance, MDR-TB and XDR-TB, respectively) in our study were close to those reported by the latest nationwide baseline survey carried out in China from 2007 to 2008 showing that the percentage of pulmonary TB patients with any resistance, MDR-TB and XDR-TB were 37·79%, 8·32%, and 0·68%, respectively [19]. This finding suggests that decreased rates of resistant-TB cases in the 309 Hospital in recent years could be associated with an overall better control of drug-resistant TB in China.
Contradictory findings regarding the association between drug-resistant TB and gender were reported. The results from a meta-analysis which reported that male sex is a determinant of drug-resistant TB in Europe [Reference Faustini, Hall and Perucci20], which is contrary to some other studies noting that female sex increased the risk for drug-resistant TB [Reference Vashakidze21–Reference Kkliiman and Altraja24]. In our study, no significant association between patients' gender and drug-resistant TB was observed. Further, we did not find an association between drug-resistant TB and sources of specimens.
The fact that patients aged 15–65 years had higher percentage of drug-resistant TB in our study is not surprising and similar results have been reported by other studies [Reference Kkliiman and Altraja24–Reference Shen27]. This finding could be related to ongoing transmission of drug-resistant TB as people aged 15–65 years are more likely to be students and commuter workers who are consistently exposed to densely populated areas.
Our study documented similar high prevalence of resistance to all four tested first-line drugs including isoniazid (30·2%), rifampin (28·4%), ethamtubol (26·3%), and streptomycin (26·7%) during 1997–2009. It is a regrettable situation that very high rates of resistance to isoniazid and rifampin were observed in our study, because these two MDR-TB-defining anti-TB drugs have played a central role in the treatment of TB, and loss of them means that only less effective and more toxic first-line and second-line drugs are available for TB treatment [Reference Espinal26]. Different patterns of resistance to first-line drugs were reported by other studies. For example, a study conducted in Iran reported higher resistance to streptomycin (33·6%) and ethambutol (27·7%) [Reference Shamaei28], while in a study carried out in Saudi Arabia, resistance to isoniazid (35·0%) and ethambutol (21·0%) was more frequent [Reference Al-Tawfiq, Al-Muraikhy and Abed29]. In another study conducted in Taiwan, the rates of resistance to ethambutol (23·4%) and isoniazid (15·4%) were higher than others [Reference Lai30]. We also observed relatively higher rates of resistance to second-line drugs tested, especially to kanamycin compared to other studies [Reference Paramasivan31, Reference Prammananan32]. Second-line drugs were available over the counter in China before 2004 and have been widely used by physicians for treating infections other than TB, this could be one of the factors contributing to high rates of resistance to them before 2004.
The prevalence of MDR-TB (19·4%) and XDR-TB (1·3%) cases in our hospital during 1997–2009 were intermediate compared to other reports. For example, the rates of MDR-TB and XDR-TB cases were 5·7% and 0·4%, respectively, from a study in Taiwan for 2000–2006 [Reference Lai30]. A study conducted in Germany for 2004–2006 reported the rates of MDR-TB and XDR-TB cases to be 4% and 0·15%, respectively [Reference Eker33]. However, the rates of MDR-TB and XDR-TB cases were as high as 43·1% and 5·5%, respectively, from a study in Georgia [Reference Vashakidze21].
Our study also documented relatively high prevalence of TB patients with mono-resistant TB or poly-resistant TB. In addition, we observed relatively higher percentage of poly-resistant TB in younger patients (⩽65 years) than older patients (>65 years). Our results suggest that high prevalence of poly-resistant TB cases, especially in younger patients warrants attention because they are as dangerous as MDR-TB cases in terms of the numbers of available efficient first-line drugs available for treatment.
We observed that the increasing trends of drug-resistant TB were reversed during the past decade, especially since 2004, in all TB cases and those who were Beijing residents. These observations were largely concurrent with TB control efforts and the overall achievements made in China. Progress in TB control had been slow before 2000 and surveillance over the next few years further revealed widespread drug-resistant TB cases including MDR-TB cases [Reference Chen1, 2, Reference Espinal26, Reference Dye34]. The increase in drug-resistant TB cases during 1997–2000 could also be linked to a malfunctioning health system during that period in China, as only 20% of TB patients treated outside the public health system took their drugs regularly in 2000, and such irregular treatment could contribute to drug-resistant TB [2]. In addition, the DOTS strategy was only implemented in 13 provinces of China between 1992 and 2001 [35], and individualized regimens were given to TB patients mainly based on the patient's financial situation instead of DST results in our hospital at that time, which could also partly account for the high prevalence of drug-resistant TB cases then. The slow-down in increasing trends of drug-resistant TB cases during 2000–2003 could be mainly associated with increased efforts made by central government such as revitalizing its TB control programme in 2000 and increased political commitment to deal with TB after realizing the severity of the situation [Reference Wang, Liu and Chine3]. Since 2004, more intensive measures were taken by central government to further strengthen its public health systems, including promoting DOTS strategy nationwide, revising laws regarding the control of infectious diseases, and starting a programme to rebuild local public health facilities and national infrastructure, etc. By 2005, the implementation of DOTS strategy was applied to 100% of counties in China and the TB treatment success rate reached more than 90% [Reference Wang, Liu and Chine3]. Beijing municipal government also increased efforts in TB control by providing free anti-TB drugs for its residents and migrants (individuals from other provinces of China who moved to Beijing) since 2006. In addition, more intensive measures have been taken in our hospital including conducting DST for all re-treatment cases and prescribing regimens consisting of at least more than three potentially effective drugs for patients based on most recent DST results available. These efforts could help further reduce drug-resistant TB cases as observed in our study in recent years. However, we also realize that despite the encouraging findings of overall decreasing trends of drug-resistant TB cases, the remaining relatively high prevalence of MDR-TB and poly-resistant TB cases still pose a challenge for TB control.
Our study has several limitations. First, the subjects of this study were from a TB referral hospital in Beijing, but since it is a major TB referral hospital receiving TB cases from Beijing and other provinces of China, the information provided from this study could be useful in devising programmatic and clinical approaches for better management of drug-resistant TB in similar settings in other provinces of China. Second, a proportion of culture-positive TB patients without a DST result might have introduced a selection bias in our study. Nevertheless, such underestimation is likely to be minimal. Third, as this study aimed to assess the patterns and trends of drug resistance in TB patients only, we did not report on risk factors and treatment outcomes.
Our study provides evidence demonstrating that the increasing trends of drug-resistant TB cases were reversed in the 309 Hospital during the past decade, but the prevalence of MDR-TB and poly-resistant TB cases remained high. Thus continuous surveillance of drug-resistant TB and better case management including provision of individual treatment regimens for TB cases based on most recent DST results should be maintained in order to prevent further development of drug-resistant TB.
ACKNOWLEDGEMENTS
This work was supported by the Key Research Program of the 309 Hospital, China (09QD01) to C.H.L.; the Knowledge Innovation Programme of the Chinese Academy of Sciences, China (KSCX2-YW-R-164), and the State Key Development Program for Basic Research of China, China (2009CB522605) to B.Z., and a grant from the Ministry of Science and Technology, China (2008ZX10005-010) to H.M.L.
DECLARATION OF INTEREST
None.







