Introduction
Land-use planning involves the allocation of land to different uses across a landscape in a way that balances values, identifying a good combination of land uses that is best able to meet the needs of stakeholders (FAO 2021). Habitat quality and ecological processes are influenced by landscape configuration, the distribution, size and abundance of forest patches (related to habitat fragmentation) and the isolation and distancing of native vegetation (Jordano et al. Reference Jordano, Bascompte, Olesen and NM2006).
Regarding landscape conservation, resources are limited (Di Minin et al. Reference Di Minin, Hunter, Balme, Smith, Goodman and Slotow2013), and protection targets (i.e., sites to be restored or protected) need to be carefully selected (Fonseca & Venticinque Reference Fonseca and Venticinque2018). In this context, the planning process to fill conservation gaps needs to take into consideration threatened and endemic species, ecosystem services and landscape features (Margules & Pressey Reference Margules and Pressey2000). This systematic conservation planning can be designed to determine priority areas for conservation purposes and protected areas.
Protected areas play an important role in biodiversity conservation, integrating elements of the landscape and allowing sustainable use of natural resources, ecosystem restoration and the provision of important ecosystem services (Silva & Prates Reference Silva and Prates2020). They are responsible for safeguarding species with high environmental requirements across a diversity of ecosystems, but alone they cannot guarantee species persistence in the long term (Sollmann et al. Reference Sollmann, Tôrres and Silveira2008). Therefore, improving connectivity among protected and unprotected areas is paramount for conservation (Castilho et al. Reference Castilho, Hackbart, Pivello and Santos2015).
Connectivity – the degree to which a landscape allows or limits species flow between vegetation fragments – can be structural, based on the number of habitat patches and their spatial configuration in a landscape (Baudry & Merriam Reference Baudry, Merriam and KF1988), or functional, which refers to the behavioural response of organisms to landscape structures (Crooks & Sanjayan Reference Crooks, Sanjayan and KR2006). In both cases, the existence of an organism’s habitat outside protected areas increases conservation values because larger populations have less extinction risk (Pimm et al. Reference Pimm, Jones and Diamond1988), species could access other habitats (Hansen & Rotella Reference Hansen and Rotella2002) and edge effects are reduced (Woodroffe & Ginsberg Reference Woodroffe and Ginsberg1998, Brashares et al. Reference Brashares, Arcese and Sam2001, Brooks et al. Reference Brooks, Mittermeier, Mittermeier, da Fonseca, Rylands and Konstant2002, Laurance Reference Laurance2009).
The Atlantic Forest biome is a globally important biodiversity hotspot (Myers et al. Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000), originally located in 17 Brazilian states, from Rio Grande do Sul to Rio Grande do Norte, and also in Argentina and Paraguay (de la Sancha et al. Reference de la Sancha, Boyle and McIntyre2021), but today supporting c. 72% of Brazilians and generating 70% of the national gross domestic product (MMA 2022). Currently, only 12.4% of the original Brazilian Atlantic Forest habitat remains (Fundação SOS Mata Atlântica & INPE 2021). This biome was the first in Brazilian history to be degraded and deforested (due to its coastal location) and the first to have a protected area and specific legislation under Brazilian Federal Law n° 11.428/2006 (Brasil 2006). Habitat loss and degradation in this biome, resulting from intense fragmentation (Ribeiro et al. Reference Ribeiro, Metzger, Martensen, Ponzoni and Hirota2009, Reference Ribeiro, Batista and Prado2011), are related to anthropogenic activity (Dean Reference Dean1996); vegetation remnants are surrounded by agricultural areas (MapBiomas 2021). One strategy to minimize human pressures on the Atlantic Forest is the creation of protected areas. However, in a future of urban expansion and increasing human requirements for natural resources (Starzynski et al. Reference Starzynski, Simões, Soares and Mendel2018), forest conservation inside protected areas is not guaranteed.
To maintain conservation values, connectivity corridors among protected and unprotected areas are of the utmost importance, and identifying habitat gaps is also necessary (Sanderson et al. Reference Sanderson, Redford, Vedder, Coppolillo and Ward2002). Structurally connected landscapes ensure ecosystem functions and resilience (Saunders et al. Reference Saunders, Hobbs and Margules1991, Lundberg & Moberg Reference Lundberg and Moberg2003, Haddad et al. Reference Haddad, Brudvig, Colbert, Davies, Gonzalez and Holt2015). Deforestation and the conversion of natural ecosystems to agricultural land and urban areas threaten protected areas, natural ecosystems and biodiversity conservation (Moraes et al. Reference Moraes, Mello and Toppa2017). Some organisms, such as large carnivores, need higher-quality environments, and protecting these species is thought to help protect other populations with less specific needs (so-called umbrella species; Begon et al. Reference Begon, Townsend and Harper2006). On the other hand, invasive species tend not to have competitors or predators in their new environments, and they usually have fewer environmental needs (Chantrey et al. Reference Chantrey, Dale, Read, White, Whitfield and Jones2014), which helps them occupy disturbed and degraded sites.
We used data on land use and land cover combined with experts’ opinions about species permeability and circuit theory to identify the connectivity opportunities in the Atlantic Forest. The study area comprised a portion of the south-east Atlantic Forest biome and two species with different environmental requirements, namely the jaguar Panthera onca, a vulnerable species with high requirements (Brasil 2022), and the wild pig Sus scrofa, an invasive species with low requirements. In a study of two Felidae species in fully protected areas in this region, Castilho et al. (Reference Castilho, Hackbart, Pivello and Santos2015) found a narrow band of landscape that was permeable to both species. We extended that analysis to all protected areas, including private protected areas and those of sustainable use, and we included an invasive species in this analysis. We hypothesized that the patchy and altered landscape of the south-east Atlantic Forest would not support the connectivity of jaguars but would facilitate that of wild pigs. We expect our data to inform landscape management strategies in the extremely fragmented Atlantic Forest.
Methods
Study site
The study was located in the Paraíba do Sul River Valley (142.34 km2; Fig. 1), which contains 36 municipalities. The region is located between São Paulo and Rio de Janeiro, Brazil’s biggest cities, with there being a current population of c. 2 million in the Paraíba do Sul River Basin (IBGE 2017). The basin region is hilly (20–45% sloped; Embrapa 1979), and it is located between two mountain ranges: the Serra da Mantiqueira and the Serra do Mar. The soil is red-yellow latosol (Lemos et al. Reference Lemos, Bennema, Santos, Iturri, Inclan, Panoso and RC1960), and the vegetation is Atlantic Forest (transition between evergreen and semi-deciduous forest) and savanna (Governo do Estado de São Paulo Reference MA2020). The climate is dry-winter subtropical (Alvares Reference Alvares, Stape, Sentelhas, Gonçalves and Sparovek2013), with an average annual temperature of 21°C and a relative humidity of c. 70% (www.cptec.inpe.br).
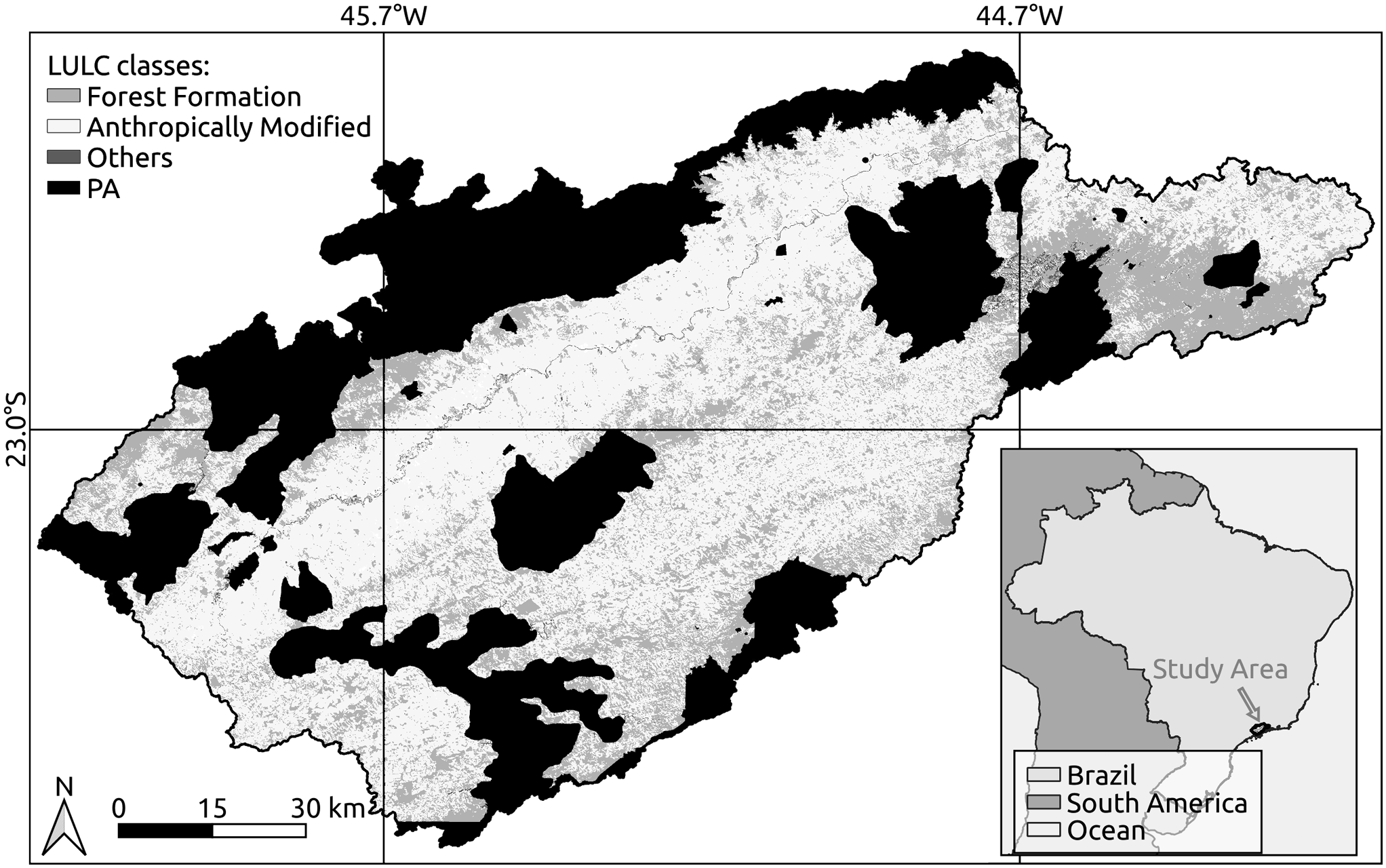
Fig. 1. Land-use and land-cover (LULC) classes of the Paraíba do Sul River Valley, São Paulo State, Brazil. Anthropogenic cover includes pasture, agriculture and urban infrastructure. PA = protected area.
The landscape in the Paraíba do Sul River Valley in São Paulo State is dominated by pasture, small fragments of secondary forest and silviculture (Eucalyptus species; Sapucci et al. Reference Sapucci, Negri, Casaca and Massi2021). Sugar cane was extensively cultivated in the 17th century and coffee in the 19th century, and urban–industrial expansion along the road–railway axis (especially around Dutra highway) gave rise to an anthropogenic landscape (Dean Reference Dean1996). Industry, especially that linked to pulp and paper production, and low-intensity pasture later took over the region, which has since become a focus of natural regeneration of the Atlantic Forest, the vegetation cover having increased by 2015 to more than 35% of the area (Numata et al. Reference Numata, Silva, Cochrane and d’Oliveira2017, Silva et al. Reference Silva, Nanni, Teodoro and Silva2017, Sapucci et al. Reference Sapucci, Negri, Casaca and Massi2021). Three different land-use and land-cover (LULC) forms can be identified (Fig. 1): Forest Formation, Anthropically Modified (forest plantation, pasture and agriculture/pasture, urban infrastructure and crops) and Others (rocky outcrop, river and lake).
In Brazil, protected areas are classified as either strictly protected, aiming to preserve nature with only indirect use of natural resources, or sustainable use, where nature conservation is compatible with sustainable use of a portion of the natural resources (Brasil 2000). The data on protected areas came from the Fundação Florestal (https://www.infraestruturameioambiente.sp.gov.br/fundacaoflorestal/), which is responsible for the management of São Paulo State protected areas, the Instituto Chico Mendes de Biodiversidade (ICMBio; https://www.gov.br/icmbio/pt-br), which is responsible for the management of national protected areas, and DataGeo (http://datageo.ambiente.sp.gov.br). The Secretaries of Environment of the municipalities of Paraíba do Sul River Valley were also contacted to identify municipal protected areas.
Our search found 50 protected areas in the Paraíba do Sul River Valley study region (Table 1). Protected areas that overlapped and were bordering others comprised a single focal node, represented by indexes (Table 1).
Table 1. Protected areas in the south-east Atlantic Forest, Paraíba do Sul River Valley and its municipalities, their size, management, conservation category and creation decrees, São Paulo State, Brazil. Numbers indicate focal nodes.

Study species
We focused on the native and threatened jaguar (P. onca: Felidae) and the invasive wild pig (S. scrofa: Suidae). The jaguar is the largest feline in the Americas (Morrison et al. Reference Morrison, Sechrest, Dinerstein, Wilcove and Lamoreux2007) and is very sensitive to habitat loss and fragmentation (Wilson & Reeder Reference Wilson and Reeder2005, Paula et al. Reference Paula, Negreiros, Azevedo, Fernandes, Stehmann and Silveira2015) because it needs large areas for survival and well-conserved forests (Borrajo et al. Reference Borrajo, Bao and Palomares2017, Diniz et al. Reference Diniz, Fischer and Aguiar2022). The species is considered Vulnerable (Ministério do Meio Ambiente 2022, Brasil 2022; albeit only Near Threatened by IUCN 2017) to the fragmentation of natural habitats, its numbers having declined in the Atlantic Forest of southern Brazil (De Angelo et al. Reference De Angelo, Paviolo, Wiegand, Kanagaraj and Di Bitetti2013) and Paraguay (Diniz et al. Reference Diniz, Fischer and Aguiar2022), justifying its use as an umbrella species (Zanin & Machado Reference Zanin, Machado and AC2014). The wild pig, a native of Eurasia, was introduced into Brazil for commercial purposes during the 1990s and quickly became invasive (Deberdt & Scherer Reference Deberdt and Scherer2007). It is currently widespread in forested habitats and in open and cultivated areas of south, central and south-east Brazil (Pedrosa et al. Reference Pedrosa, Salerno, Padilha and Galetti2015).
Mapping
We initially employed a 30m spatial resolution map provided by the MapBiomas Project (Souza et al. Reference Souza, Shimbo, Rosa, Parente, Alencar and Rudorff2020; from Annual Series of Land Use and Land Cover of Brazil – Collection 6, accessed on 17 November 2020 through www.mapbiomas.org) of the LULC classes in the study area in 2019 (Fig. 2). The 15 LULC classes in the study area were grouped into eight uses by relative importance within the study region. In addition, agriculture and pasture uses were grouped due to the similar permeability of these land covers to animals.

Fig. 2. Method flowchart. LCP = least cost path; LULC = land use and land cover; PA = protected area.
The permeability values relative to each LULC class were derived from the knowledge of three experts (McRae Reference McRae2006) with known experience in the field of the habitat needs and landscape configurations of both wild pig and jaguar species in the Atlantic Forest and in Paraíba do Sul River Valley. These experts were a zoologist and conservation biology professor at a local university, a researcher and non-governmental organization technician working on fauna management in the region and a specialist in the region’s fauna. They weighted the LULC classes’ permeability for the two species with values between 0 and 100, where 100 implied the least permeability (higher resistance to animal movement) and 0 the most permeability (lower resistance to animal movement). From the suggested permeabilities of each species and LULC type, we computed the average and variation values (Fig. 3). In general, resistance to wild pigs was lower than that to jaguars, but forest formations were permeable to both species (Fig. 3), as in other studies (Pedrosa et al. Reference Pedrosa, Salerno, Padilha and Galetti2015, Borrajo et al. Reference Borrajo, Bao and Palomares2017, Diniz et al. Reference Diniz, Fischer and Aguiar2022). Pasture and agriculture had higher permeability for wild pigs (Pedrosa et al. Reference Pedrosa, Salerno, Padilha and Galetti2015), but jaguars could also use these land covers to search for prey and move between forest patches (Paula et al. Reference Paula, Desbiez, Beisiegel, Campos, Sana and Moraes2013).

Fig. 3. Average permeability values of the different land uses and land covers (LULCs) for jaguars (Panthera onca, circles) and wild pigs (Sus scrofa, triangles) and the respective standard deviation values based on expert opinion.
We used isolation-by-resistance and least-cost-corridor analyses to determine the level of connectivity of the protected areas for the two focal species. The isolation-by-resistance analysis was performed using the software Circuitscape 4.0 (McRae et al. Reference McRae, Dickson, Keitt and Shah2008), where the LULC maps and resistance values were the inputs to generate current maps of the potential paths for jaguars and wild pigs. Due to the dimensions of the study area, in order to alleviate the computational cost, the LULC input map was subsampled to 60 m, and four-neighbour spatial interaction was employed to compute the resistance maps. The performed subsampling and spatial interaction type do not affect the results of analyses of large areas (McRae et al. Reference McRae, Dickson, Keitt and Shah2008).
The Circuitscape methodology uses points of analysis, not regions. All of the area inside focal nodes was defined as 0 resistance, and it was thus possible to calculate each focal node as an area. We chose the one-to-all mode (McRae et al. Reference McRae, Dickson, Keitt and Shah2008), where the connectivity was calculated between all focal regions. In each interaction, one focal region was connected to a source, which in this study represented all other focal regions, while other places were considered inactive. This analysis showed the relative value of each grid cell for providing connectivity among protected areas and identified the routes facilitating or inhibiting species movement among the focal areas (McRae et al. Reference McRae, Dickson, Keitt and Shah2008). Resistance isolation used circuit theory to predict connectivity in heterogeneous landscapes. This theory includes a random basis for assessing multiple dispersion contributions (Castilho et al. Reference Castilho, Hackbart, Pivello and Santos2015). In each interaction, a protected area was connected to a source, while the other places were considered inactive.
The least-cost-path (LCP) corridor analysis performed with the algorithm proposed by Shirabe (Reference Shirabe2015) available for QGIS showed the relative value of each raster cell in relation to connectivity between focal areas, indicating what will facilitate or inhibit the movement between protected areas. The cost raster layer used was the one generated from Circuitscape. Origins and destinies were created for each focal area to generate the paths by the LCP approach. All corridors were clumped to underline the connections that are used more than once, identifying regions where the loss of a small area could harm landscape connectivity.
Results
The landscape configuration in the Paraíba do Sul River Valley showed better connectivity between protected areas for wild pigs than for jaguars (Fig. 4). Although differences were subtle, they related to the human-altered environments being less permeable to jaguars than to wild pigs (Fig. 3).
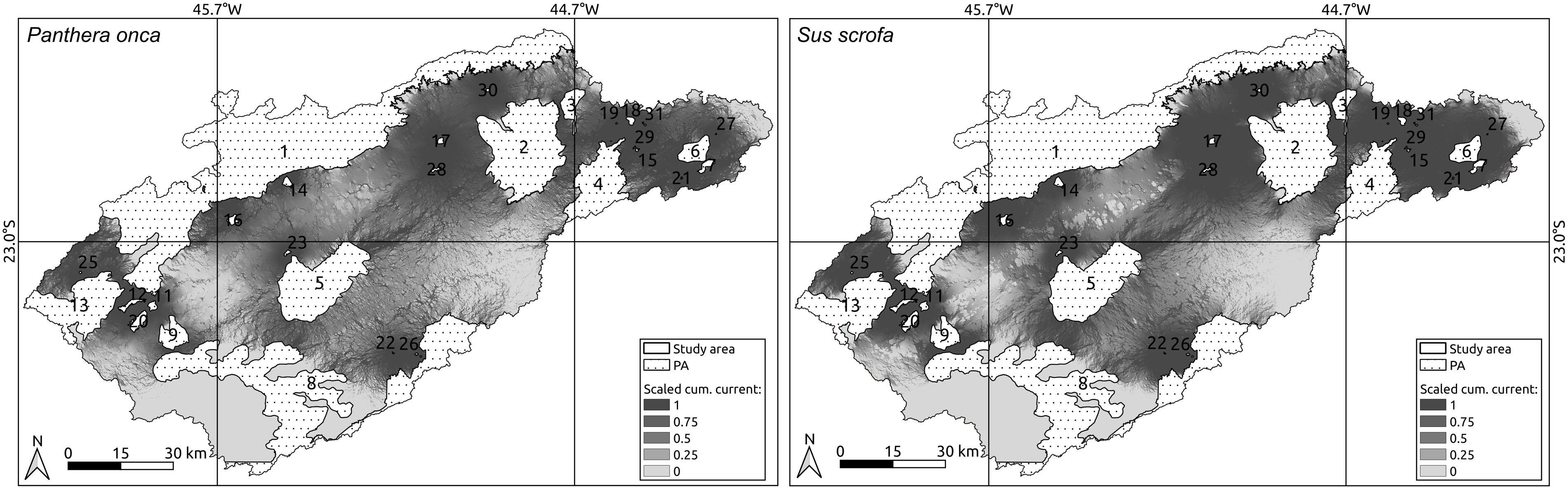
Fig. 4. Landscape connectivity for jaguars (Panthera onca, left) and wild pigs (Sus scrofa, right) showing connectivity paths among protected areas (PA) in the Paraíba do Sul River Valley. The darker shades correspond to areas most permeable to animal movement and numbers correspond to index values in Table 1. The isolation-by-resistance analyses was performed using the software Circuitscape 4.0, where the land-use and land-cover maps and resistance values (given by the experts) were the inputs to generate current maps of the potential paths for jaguars and wild pigs (designated under ‘Scaled cum. current’ in the maps).
The isolation-by-resistance and least-cost-corridor results indicated that forest remnants conserved by protected areas (Table 1) are still connected by a permeable landscape for both jaguars and wild pigs (Figs 4 & 5), but not across the whole territory. Although the studied landscape has more than 887.43 km2 of fully protected areas and 5,085.85 km2 of sustainable use protected areas, allowing species movement in a south–north direction in Serra do Mar and Serra da Mantiqueira, the links between Serra do Mar and Serra da Mantiqueira in a south-east–north-west direction are only possible through Federal APA Mananciais do Paraíba, which, despite being a focal point of connection, had a lower level of protection (Figs 4 & 5). In addition, although the protected areas covered 5,953.28 km2 (41.82% of the study site), many of them overlapped completely or partially, resulting in only 2,054.00 km2 of protected area (14.43% of the study site).
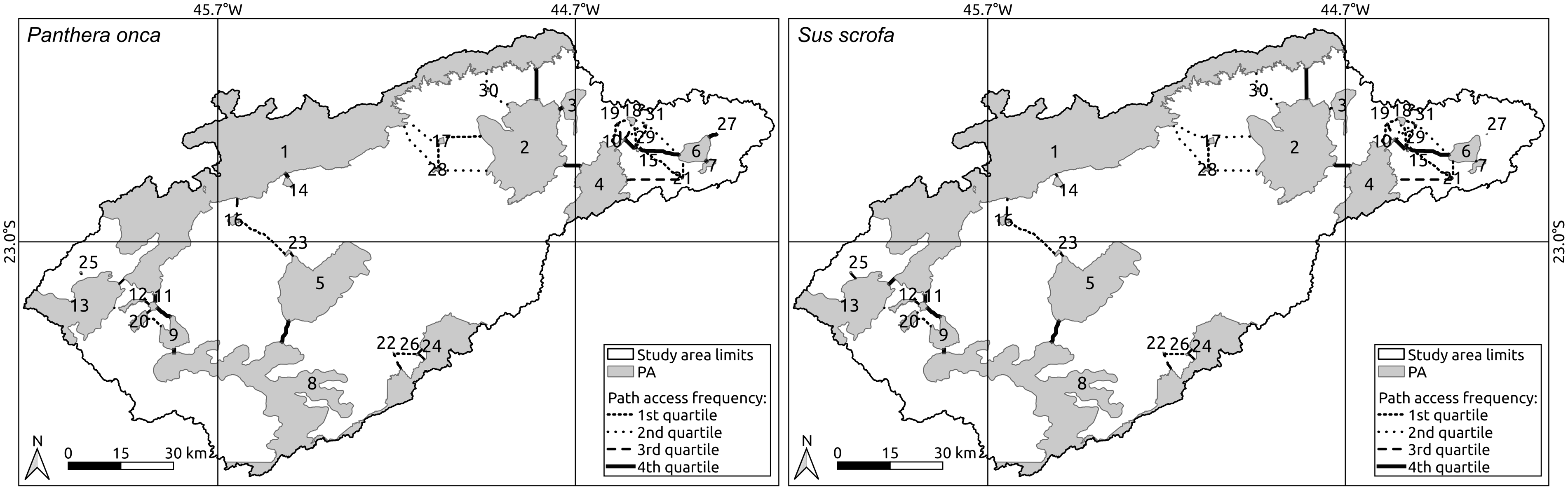
Fig. 5. Least cost path for jaguars (Panthera onca, left) and wild pigs (Sus scrofa, right) among protected areas (PAs) in the Paraíba do Sul River Valley. The fourth quartile (solid black line) represents the most used corridors and the numbers correspond to the index values in Table 1.
The first quartile of the LCP indicates the 25% of ways that had been less used, while the fourth quartile represents the 25% of paths that were being used most to connect protected areas, suggesting that they are vital to maintaining landscape connectivity (McRae et al. Reference McRae, Dickson, Keitt and Shah2008). Among fourth-quartile connections (solid pathways in Fig. 5), focal nodes 4 (Serra da Bocaina National Park), 1 (25 protected areas altogether, which comprise the Mantiqueira Mosaic), 2 (APA Paraíba do Sul and APA Silveiras), 6 (APA Paraíba do Sul), 8 (Serra do Mar State Park and APA Paraíba do Sul), 9 (APA Paraíba do Sul) and 11 (APA do Banhado and Banhado Natural Municipal Park) are fundamental to connectivity across the Paraíba do Sul River Valley landscape. In addition, private reserves (Reserva Particular do Patrimônio Natural; RPPNs), which tend to be smaller, substantially contributed to landscape connectivity, especially in the north-east, close to Serra da Bocaina National Park. There was no preferential connection between focal nodes 5 (part of APA Paraíba do Sul) and 2 (part of APA Paraíba do Sul and APA Silveiras; Fig. 5) because the LULC between them is mainly urban (around Dutra highway).
Despite landscape connectivity differing for wild pigs and jaguars, the LCP did not show large differences between the two species (Fig. 5) because wild pigs would search for the easiest way to cross the landscape, which amounts to the most vegetated areas, although they can also cross open and deforested sites.
Discussion
We found potential connectivity for both jaguars and wild pigs, but there were more connectivity opportunities for wild pigs. The connection between Serra do Mar (and Parque Estadual Serra do Mar or Serra do Mar State Park) and Serra da Mantiqueira (Mantiqueira Mosaic) is narrow but possible to traverse through some protected areas of sustainable use and private reserves, highlighting the importance of these to structural landscape connectivity for these species in this region. The same connectivity that allows the transit of jaguars, a native species with high environmental requirements, can also facilitate the movement of the invasive wild pigs through the landscape, which is worrisome.
In the region, fully protected areas – and even those of sustainable use – are usually in mountainous locations and comprise land of low economic value. However, most of the landscape is hilly and covered by pasture and agriculture (50%), while only 36% is forest formation. In the last 35 years, demographic shifts and land abandonment are allowing some pasture areas to return to forest (Silva et al. Reference Silva, Nanni, Teodoro and Silva2017, Sapucci et al. Reference Sapucci, Negri, Casaca and Massi2021), which might explain the observed connectivity for jaguars, but this process is even better for wild pigs. The region is also the location of several restoration projects (www.observatoriodarestauracao.org.br) and payment for ecosystem service programmes around protected areas (such as Conexão Mata Atlântica; São Paulo Reference Paulo2021) that could improve connectivity in the future. Since the implementation of SNUC (Brasil 2000), a law that creates, protects and manages protected areas, there has been increasing concern about preventing the isolation of protected areas. We believe that if buffer zones of protected areas were fully respected as a conservation goal, the landscape connectivity of this region would be significantly greater.
The LCP and Circuitscape analyses complemented each other, because the former assumes that a species has perfect knowledge of the landscape, while the latter considers that animals cannot plan for even one step ahead in their transitions (Carroll et al. Reference Carroll, McRae and Brookes2011). We found differences in landscape connectivity for the two species when considering the Circuitscape analysis, but not when we used the LCP analysis. Wild pigs have a more permeable landscape to move through, as their environmental requirements are not as high as those of the jaguar. Jaguars requires well-conserved forest cover (Borrajo et al. Reference Borrajo, Bao and Palomares2017, Diniz et al. Reference Diniz, Fischer and Aguiar2022), and this highlights the need for protected areas and forest remnants in the region in order to have enough habitat for this species, as well as for other species with less specific needs (RBMA 2022). Wild pigs, on the other hand, are among the most invasive alien species in the world (Global Invasive Species Database 2022). They cause damage to native fauna and flora and to crops, and they affect ecological processes, in addition to transmitting several diseases. Due to wild pigs’ low level of environmental requirements, this species could travel everywhere in the region, being constrained only by rock formations and water bodies.
The Paraiba do Sul River Valley has many silviculture areas (mainly Eucalyptus spp.), and jaguars transit these sites (Castilho et al. Reference Castilho, Hackbart, Pivello and Santos2015). However, the experts consulted here did not point to silviculture as a permeable LULC for this species. The jaguar resistance values provided by the experts diverged regarding agriculture and pasture. Therefore, the results among the given resistance numbers for the jaguar in this LULC reached a low baseline (high permeability). The expert consultation in this study was crucial to it being able to attribute resistance values that reflect reality. The experts were chosen carefully, and a standard deviation was calculated to evaluate resistance value variations among them. Wild pig values were most consistent among experts (variation was as low as 10.52%), while jaguar values were considered of medium to high consistency (26.12%), which gives credibility to the analysis performed.
Jaguars evidently prefer to use forested areas or other natural formations, avoiding anthropized areas (Paviolo et al. Reference Paviolo, De Angelo, Ferraz, Morato, Pardo and Srbek-Araujo2016, Thompson et al. Reference Thompson, Morato, Niebuhr, Alegre, Oshima and de Barros2021). They might in addition use pasture and agriculture areas to search for prey, especially the predation of livestock (Thompson et al. Reference Thompson, Morato, Niebuhr, Alegre, Oshima and de Barros2021). Our results do not imply that jaguars would inhabit agriculture and pasture locations but that they could potentially move (e.g., higher resistance values for Paraiba do Sul anthropized LULCs for jaguars) in a landscape that has been considered a forest and landscape restoration hub. In addition, jaguars are not commonly reported in the region (the last survey occurred in 2012 in Bocaina National Park), and this study aimed to use them as an umbrella species. However, our results could also be seen as highlighting ways of improving the habitat for Felidae species, especially regarding critical gaps and corridors for jaguar movement.
This study also highlights the importance of private protected areas, as the study site contained 42 of these. Although they are usually small in size, a network of these might promote connectivity in a landscape, as has been shown here. Public policies should encourage landowners to create more private reserves, but also a more forested landscape could be achieved through agroforestry systems (Santos et al. Reference Santos, Herrmann, Mieske, Krag, Haase and Stepputtis2018, Rocha et al. Reference Rocha, Massi and Mendes2020) and other agricultural conservation practices, avoiding and/or decreasing edge effects and inbreeding depression.
Our research highlights significant gaps in our understanding of the connectivity of protected areas; these should be targets of restoration and public conservation policies in this biodiversity hotspot. Yet, while knowledge of the connections between protected areas improves and contributes to the conservation of biodiversity, these connections also facilitate the transit of exotic species such as wild pigs (Glen et al. Reference Glen, Pech and Byrom2013). How the conservation of jaguars can be implemented while preventing the spread of an invasive species is a challenge for managers and decision-makers.
Acknowledgements
We thank Felipe Pedrosa (Mão na Mata – Manejo e Soluções Ambientais), Adriana Prestes and Júlio Cesar Voltolini (Universidade de Taubaté), the experts that made this study possible.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
The authors declare none.
Ethical standards
None.









