Introduction
Radioactive-waste disposal facilities use many types of materials as engineered barriers to isolate the radioactive waste, including cementitious materials, carbon steels, and bentonite (Sellin & Leupin, Reference Sellin and Leupin2014). Alkaline conditions can be produced at radioactive-waste disposal facilities by the alteration of cementitious materials and iron corrosion. For example, cementitious materials, such as ordinary Portland cement (OPC) or low-pH cement, produce alkaline porewaters with a pH range of 10–13 (García Calvo et al., Reference García Calvo, Hidalgo, Alonso and Fernández Luco2010), whereas a mixture of iron powder and water produces a solution with an approximate pH of 11 (Kaufhold et al., Reference Kaufhold, Dohrmann and Weber2020). Although the Fe or Mg content in these alkaline radioactive waste disposal systems is not large, the potential formation of Fe- or Mg-bearing clays is discussed actively. Specifically, several types of Fe- or Mg-bearing clays are known to be formed by the interaction between cementitious materials and bentonite (e.g. Fernández et al., Reference Fernández, González-Santamaría, Angulo, Torres, Ruiz, Turrero and Cuevas2018; Yokoyama et al., Reference Yokoyama, Shimbashi, Minato, Watanabe, Jenni and Mäder2021), cementitious materials and clay rocks (e.g. Dauzeres et al., Reference Dauzeres, Achiedo, Nied, Bernard, Alahrache and Lothenbach2016; Lerouge et al., Reference Lerouge, Gaboreau, Grangeon, Claret, Warmont, Jenni and Mäder2017), and iron and bentonite (e.g. Wilson et al., Reference Wilson, Cressey, Cressey, Cuadros, Ragnarsdottir, Savage and Shibata2006). As the period for the safety assessment of the radioactive-waste disposal is rather extensive, natural analogue (NA) studies, in addition to the laboratory and in situ experiments described above, remain beneficial. The study of NA involves the observation of systems in nature which form analogues with the phenomena concerned with the disposal of radioactive waste. To increase the limited number of studies investigating NA, it has been suggested that the discipline should be pursued vigorously, taking a stance which allows for system analogues to be localized (Yoshida et al., Reference Yoshida, Kitayama, Sato and Kobayashi2010). In this context, understanding the formation reaction of secondary Fe- or Mg-bearing clays during natural, long-term alkaline interactions occurring in various geological settings would be useful, even if they were not the alteration of the constituent materials in radioactive waste disposal, to predict potential secondary minerals formed by the long-term alteration in radioactive-waste disposal.
Near the Troodos ophiolite in Cyprus, Fe-bearing palygorskite was formed by interaction between bentonite and alkaline water over a time span of 105–106 y (Milodowski et al., Reference Milodowski, Norris and Alexander2016). Similarly, the interaction between bentonite and alkaline water at the Saile mine in the Philippines produced Fe- and Mg-bearing smectites (Fujii et al., Reference Fujii, Yamakawa, Shikazono and Sato2014). In the Denizli Province of Turkey, Akbulut and Kadir (Reference Akbulut and Kadir2003) reported the formation of Fe-bearing palygorskite, sepiolite, and saponite in alkaline waters. Furthermore, Furquim et al. (Reference Furquim, Barbiéro, Graham, de Queiroz Neto, Ferreira and Furian2010) discovered Fe-illite and glauconite with amorphous phases in saline lakes in the Pantanal wetland of Brazil. Chrysotile and poorly crystalline chrysotile-like phases were reported as precipitates from alkaline water at near-surface or surface environments in New Zealand (Craw et al., Reference Craw, Landis and Kelsey1987) and Japan (Nishiki et al., Reference Nishiki, Sato, Katoh, Otake and Kikuchi2020).
In addition to these previous studies, the widespread distribution of uncommon Fe- and Mg-bearing clays under alkaline conditions at Narra in Palawan, the Philippines, were recently reported (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). Although these clays have been reported to be authigenic and formed under alkaline conditions, their nature remains uncertain (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020) as shown in the next section below. The current study aimed to establish the structural and chemical characteristics and to understand the formation pathways of Fe- and Mg-bearing clays in alkaline fluids in the Narra District of the Philippines to add to existing knowledge regarding the naturally occurring Fe- and Mg-bearing clays under alkaline conditions.
Site Description and Previous Studies at The Site
The geological setting of the Narra District of the Philippines is dominated by the Palawan ophiolite, which comprises the Beaufort Ultramafic Complex, Stavely Gabbro, and Espina Formation (Aurelio et al., Reference Aurelio, Forbes, Taguibao, Savella, Bacud, Franke, Pubellier, Savva, Meresse, Steuer and Carranza2014). Because the generation of alkaline water has been reported frequently in the ultramafic rocks (Barnes et al., Reference Barnes, O'Neil and Trescases1978), this study site was selected in order to understand geochemical reactions under natural alkaline conditions.
The sampling site comprises an alluvial fan channel on a gentle slope of the Palawan ophiolite basement (Fig. 1a). Eight trenches (T1–T8) and four drill holes (DH1–DH4) were excavated at depths below the surface ranging from 0 to 16 m (Fig. 1b). Clastic sediments, which overlaid the basement (Fig. 1a), were derived from serpentinized rocks and gabbro from the Palawan ophiolite (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). The deposition of the sediments was determined to have been initiated 15,000 y ago based on 14C dating of humin in the sediments (Table 1; Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020), and the depositional environment of the sediments probably changed from seawater to brackish water and further to freshwater over time (RWMC, 2017; Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). The sediments interacted with alkaline (pH 11) seepage, which was produced by the geochemical reactions of meteoric water with the Palawan ophiolite (RWMC, 2016, 2017, 2018; Shimbashi et al., Reference Shimbashi, Sato, Yamakawa, Fujii and Otake2018). After deposition, a carbonate layer was formed on the surface through the mixing of alkaline water and surface water (Shimbashi et al., Reference Shimbashi, Sato, Yamakawa, Fujii and Otake2018).

Fig. 1. a Conceptual diagram of the sample site (modified from Shimbashi et al. (Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020)); and b aerial map showing the location of trenches and drill holes: the numbers in parentheses represent the total depths of the trenches and drill holes from the surface
Table 1 Sample descriptions and d values obtained from the XRD analyses

*indicates data sourced from Shimbashi et al. (Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). Values for carbon isotope measurements were obtained from humin in the sediments.
The clastic sediments in this area comprised predominantly a mineral with a broad X-ray diffraction (XRD) peak at 14 Å (14 Å phase), orthopyroxene, amphibole, serpentine, talc, chlorite, calcite, and 14 Å tobermorite (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). Newly formed phases in the sediments included Fe- and Mg-bearing clays (i.e. 14 Å phases) and 14 Å tobermorite. The Fe- and Mg-bearing clays were categorized previously into three types based on XRD analysis (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). Type-I exhibited trioctahedral characteristics based on the d 060 position, and its expandability after ethylene glycol (EG) treatment and its dehydration behavior (Fig. 2a) differed from those of smectite and chlorite. Type-II exhibited a nature intermediate to that of Type-I and trioctahedral smectite, according to the expandability after EG treatment and dehydration behavior (Fig. 2b and c). In contrast, Type-III displayed dioctahedral characteristics based on the d 060 position and exhibited expandability after EG treatment similar to smectite, although the dehydration state implied the presence of other phases of smectite (Fig. 3). The uncertainty of the characterization above led to labeling both Types-I and II as iron-magnesium-silicate-hydrates (F-M-S-H), and Type-III as a nontronite-like mineral (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020).

Fig. 2. XRD profiles of F-M-S-H before and during heat treatment at 250°C; a DH1-7; b T4-3; c DH1-4; data sourced from Shimbashi et al. (Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020)

Fig. 3. XRD profiles of nontronite-like mineral before and during heat treatment at 250°C; a DH4-9; b DH3-1; c T7-7; data sourced from Shimbashi et al. (Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020)
The F-M-S-H and 14 Å tobermorite were recognized at shallower sampling depths in the middle of the fan, in the sediments deposited in freshwater (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). The nontronite-like mineral was observed at a deeper sampling depth in the middle of the fan and at a shallower sampling depth at the fan toe side (Fig. 1b), in the sediments deposited in seawater. The correlation between the distribution of secondary minerals and the depositional environment suggested that the depositional environment determined the species of secondary minerals. Specifically, the F-M-S-H and 14 Å tobermorite were considered to have been formed through the interaction between clastic sediments and alkaline seepage without seawater infiltration, whereas the nontronite-like mineral was formed presumably by a similar interaction with the involvement of seawater (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020).
Materials and Methods
Samples, which were numbered successively from bottom to top, were selected to represent the sediment from all the trenches and drill holes except for the T8, DH2, and T7-2 samples. This is because the spatial distribution of primary and secondary minerals, as well as depositional age and depositional environment in those sediment samples, had already been examined thoroughly in a previous study (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). In the present study, a <2 μm fraction from the 18 samples, including the seven samples analyzed by Shimbashi et al. (Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020), were selected and examined to understand the structural and chemical characteristics of the Fe- and Mg-bearing clays. To obtain the <2 μm solid fraction, the sediment samples were dispersed in distilled water and centrifuged for 4 min at 1000 rpm (190×g) using an ST-720M rotor and centrifuge (Kubota 4000 centrifuge, Tokyo, Japan) to form a <2 μm supernatant. The supernatant was further centrifuged for 10 min at 14,000 rpm (27,172×g) using an NA-8 rotor and centrifuge (TOMY Suprema21, Tokyo, Japan). The <2 μm solid fraction obtained was dried in a vacuum.
Following the drying of the solid fraction, an XRD analysis of the 18 oriented samples was conducted using an X-ray diffractometer (Rigaku Smart Lab, Tokyo, Japan) with CuKα radiation set to 45 kV and 30 mA, at a scanning speed of 5°2θ/min, and a scanning step size of 0.01°2θ. The following slits were applied: divergence slit of 1/12°2θ, receiving slit of 20 mm, and a solar slit of 2.5°2θ. For the preparation of the oriented sample, 10 mg of the <2 μm samples was resuspended in distilled water and transferred onto a glass slide for the XRD analysis. After analysis, the oriented samples were further solvated with EG vapor at 60°C for >12 h, after which the XRD analysis was conducted on the EG-solvated samples using the same method as for the oriented samples. The randomly oriented samples of the <2 μm fraction were measured using XRD to observe the d 060 of the Fe- and Mg-bearing clays. The XRD analyses for the randomly oriented samples were similarly conducted with CuKα radiation set to 45 kV and 30 mA, at a scanning speed of 0.3°2θ/min, and a scanning step size of 0.01°2θ. The following slits were also applied: divergence slit of 1/12°2θ, receiving slit of 20 mm, and a solar slit of 2.5°2θ.
A chemical analysis was conducted for 10 samples selected from the XRD results to represent the diversity of the samples. The Fe- and Mg-bearing clays were analyzed chemically using field emission scanning electron microscopy (FESEM) with energy dispersive X-ray spectroscopy (FESEM-EDS; JEOL JSM-7001M, Tokyo, Japan). The chemical composition of ~50 points was analyzed for each sample, and the median values of elemental ratios in each sample were compared.
57Fe Mössbauer spectroscopy was conducted at room temperature and 77 K using 57Co in metallic Rh as a gamma-ray source. The <2 μm fractions in the DH1-7, DH1-4, and DH4-9 samples were selected as representative samples of F-M-S-H at shallower sampling depths, F-M-S-H at moderate sampling depths, and nontronite-like mineral, respectively (Table 1). The velocity scale was calibrated using a spectrum of α-Fe measured at room temperature as well as at 77 K. The spectra were fitted to a sum of doublets using a least-squares computer program and were characterized by three doublets. Isomer shifts (IS) are sensitive to the oxidation state and the coordination number of Fe ions and were allotted regarding α-Fe. Quadrupole splitting (QS) is a measure of the electric field gradient at the 57Fe nucleus.
The infrared (IR) spectra were obtained using the three samples subjected to Mössbauer spectroscopy. The <2 μm samples were examined as KBr pellets which were dried for 1 day at 110°C and cooled in a desiccator filled with silica gel before analysis. The spectra were recorded from 100 scans at a resolution of 4 cm–1, between 3800 to 370 cm–1, using a Ge/KBr beam-splitter and a DLATGS detector on a Fourier-transform infrared spectrometer (JASCO FT/IR-6100, Tokyo, Japan).
The additional detailed analyses described below were conducted for F-M-S-H in the DH1-7 samples. This was because F-M-S-H in the DH1-7 samples (Type-I) exhibited a behavior in the XRD analysis during heating at 250°C that was different from Type-II F-M-S-H and the Type-III nontronite-like mineral (Figs 2 and 3; Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). For the purpose of dissolving the interlayer hydroxide sheets between tetrahedral-octahedral-tetrahedral (TOT) layers (Rich & Bonnet, Reference Rich and Bonnet1975), an acid treatment was performed for the <2 μm fractions of the DH1-7 samples. Although the acid treatment conditions differed from those used in a previous study (Rich & Bonnet, Reference Rich and Bonnet1975), it was conducted at room temperature (~23°C) by adding 50 mg of the <2 μm fraction to 5 mL of a 0.1 M HCl solution and shaking for 30 min. To understand species of interlayer cations, an alkylammonium treatment was conducted by adding 15 mg of the <2 μm fraction to 5 mL of a 0.1 M dodecylamine hydrochloride (nC = 12) solution and incubating at 65°C for 2 days. The suspension for the alkylammonium treatment was separated using centrifugation, and the solid sample was redispersed in 5 mL of a 0.1 M dodecylamine hydrochloride solution and incubated at 65°C for 3 days. The acid-treated or alkylammonium-treated <2 μm fractions were washed with 80 or 100% ethanol several times until the supernatants were chloride free. The oriented sample was prepared using the washed samples. The acid-treated sample was solvated with EG vapor at 60°C for over 12 h, whereas the alkylammonium-treated sample was dried at 65°C for over 1 h before analysis. The XRD analysis of those samples was conducted using the same method as that used for the oriented samples.
The acid-treated <2 μm fraction was also examined by the XRD analysis with heat treatment. Because the sample amount was limited, the heat treatment was performed differently from that of a previous study, which analyzed the <2 μm fraction without acid treatment (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). Specifically, 10 mg of the acid-treated <2 μm fraction was resuspended in distilled water, transferred onto a silica glass slide, and dried. The acid-treated sample was analyzed using XRD, like the oriented samples described above. After the XRD analysis, the acid-treated sample was heated at 250°C for 2 h in a muffle furnace, and the sample was cooled in a desiccator filled with silica gel. The XRD analysis of the heated sample was conducted similarly to the oriented samples within 10 min of heating.
The chemical components of the acid-treated or alkylammonium-treated <2 μm fractions were obtained using FESEM-EDS in the same manner as described above. The variation in chemical composition before and after the acid or alkylammonium treatments were displayed using box-and-whisker plots.
Results
XRD Analysis
The <2 μm fractions were composed predominantly of 14 Å phases with trace amounts of serpentine (Fig. 4a, c, and e). The d 001 of the 14 Å phases shifted to a lower angle following EG treatment; it could not be determined, however, if the XRD reflection linked to the 001 reflection followed rationality in some samples, due to a small peak intensity (Fig. 4a). All of the 14 Å phases, except for samples T4-1 and DH3-9, were divided into F-M-S-H and nontronite-like mineral categories (Table 1 and Fig. 4), based on the values for d 001 after EG treatment and d 060 of the 14 Å phases (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). Sample T4-1 represented a mixture of F-M-S-H and nontronite-like mineral (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020), probably the same for DH3-9. The distribution of F-M-S-H and nontronite-like mineral was identical to the trend reported in a previous study (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020), despite the increased number of samples analyzed in the current study. Moreover, the d 001 after EG treatment of the F-M-S-H phases increased with increasing sampling depth, whereas that of all nontronite-like minerals showed the same expandability despite differences in the sampling depth (Fig. 5).
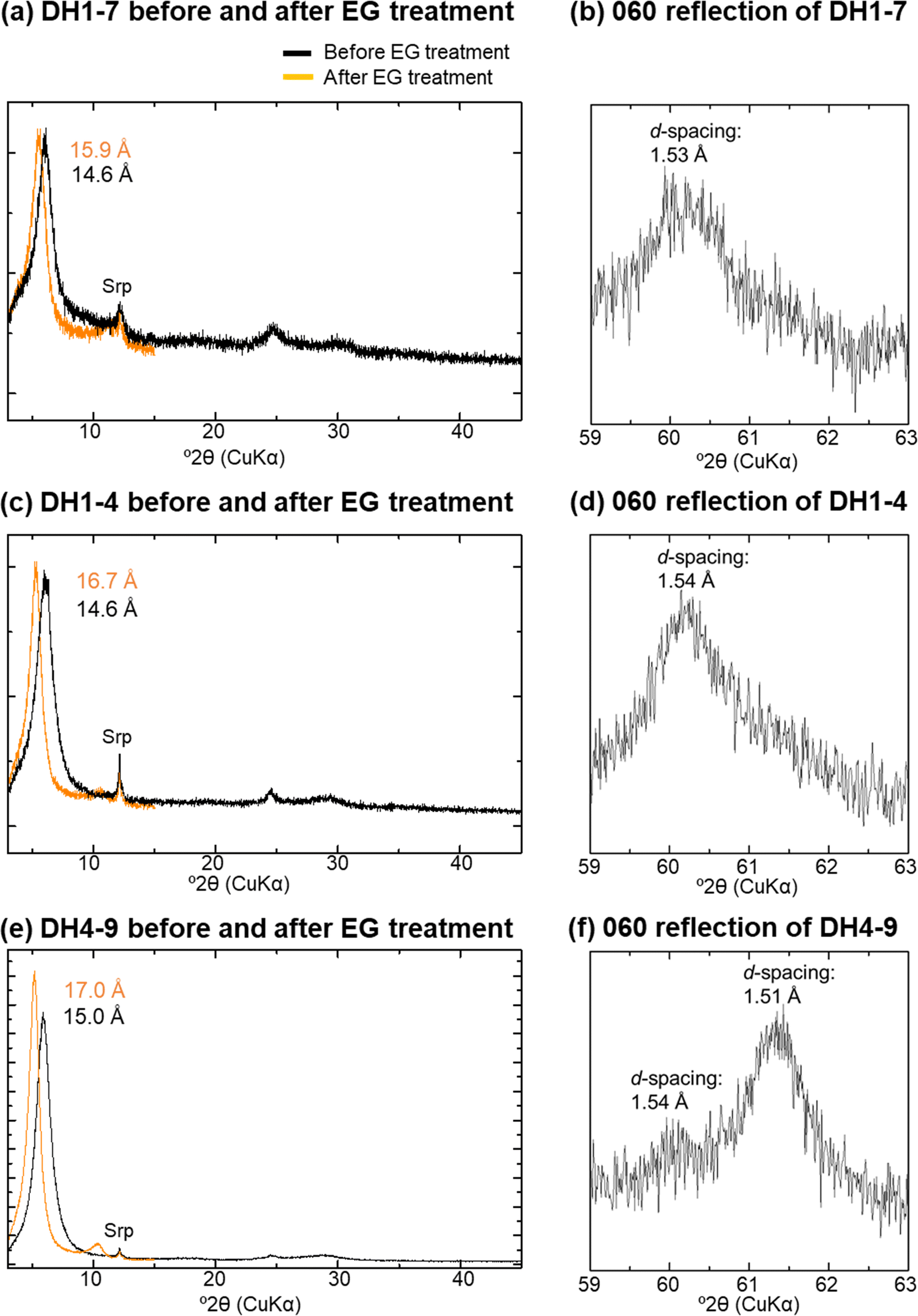
Fig. 4. XRD profiles before and after EG treatments of oriented samples and 060 reflection of randomly oriented samples; a and b: DH1-7; c and d: DH1-4; e and f: DH4-9; Srp: serpentine; data sourced from Shimbashi et al. (Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020)

Fig. 5. d 060 and d 001 after EG treatment at different sampling depths
Chemical Composition
The chemical composition of both F-M-S-H and the nontronite-like mineral consisted mostly of Fe, Mg, Si, Ca, and Al ions. In the ternary diagram of Al-Mg-Fe, F-M-S-H plotted in a similar position to that of the nontronite-like mineral (Fig. 6a). Both the F-M-S-H and nontronite-like mineral were rich in Fe and Mg, and poor in Al. However, clear differences in the chemical composition between F-M-S-H and the nontronite-like mineral were recognized in the ternary diagram of Ca–Si–Fe+Mg+Al (Fig. 6b). F-M-S-H exhibited a chemical composition slightly richer in Ca and slightly poorer in Si than that of saponite, the trioctahedral smectite. The nontronite-like mineral was similar, in terms of chemical composition, to the ideal nontronite, the dioctahedral smectite.

Fig. 6. Chemical compositions in ternary diagram: a ternary diagram of Al–Mg–Fe; b ternary diagram of Ca–Si–Fe+Mg+Al
The median values of the (Fe+Mg)/(Si+Al) ratio, the (Fe+Mg+Al)/Si ratio, the Fe/Mg ratio, and the Al/(Si+Al) ratio of the F-M-S-H decreased continuously with increasing sampling depth, whereas the ratios of all nontronite-like minerals displayed relatively similar values (Fig. 7a–d). Furthermore, the median value of the Ca/(Si+Al) ratio of the F-M-S-H ranged between 0.08 and 0.15, while that of the nontronite-like mineral exhibited lower values ranging between 0.02 and 0.06 (Fig. 7e and Table 2).

Fig. 7. Chemical compositions at different sampling depths: a (Fe+Mg)/(Si+Al) ratios; b (Fe+Mg+Al)/Si ratios; c Fe/Mg ratios; d Al/(Si+Al) ratios; e Ca/(Si+Al) ratios
Table 2 The median values of chemical composition ratios

Mössbauer Spectroscopy Analysis
The Mössbauer spectra of the F-M-S-H were similar, regardless of sampling depth (Fig. 8a, b, d, and e). They displayed two dominant Fe3+ doublets (Tables 3 and 4). The IS and QS values of the Fe3+ inner-doublet at room temperature (Table 3) exhibited similar values to that of Fe3+ in the octahedral site of the TOT layer for nontronite, which recorded an IS and a QS of 0.38 and 0.63 mms–1 at room temperature, respectively (Johnston & Cardile, Reference Johnston and Cardile1985). Additionally, similar values of Fe3+ were shown in the octahedral interlayer hydroxide sheet of chlorite and recorded an IS and a QS of 0.34 and 0.69 mms–1 at room temperature, respectively (Czaja et al., Reference Czaja, Kadziołka-Gaweł, Lisiecki, Bodył-Gajowska and Mazurak2014). On the other hand, the Fe3+ outer-doublet was characterized by high QS values (Tables 3 and 4). The IS and QS values of the Fe3+ outer-doublet (Tables 3 and 4) showed similar values of iron in octahedral sheets in the oxidized chlorite, which recorded an IS of 0.36 mms–1 and a QS of 1.04 mms–1 at 300 K, respectively, as well as an IS of 0.41 mms–1 and a QS of 0.94 mms–1 at 4 K (Kodama et al., Reference Kodama, Longworth and Townsend1982). Furthermore, similar values were found in the oxidized trioctahedral smectite which was previously ferrous iron-bearing (e.g. ferrian saponite), with an IS and a QS of 0.36–0.38 mms–1 and 1.31–1.38 mms–1 at room temperature, respectively (Treiman et al., Reference Treiman, Morris, Agresti, Graff, Achilles, Rampe, Bristow, Ming, Blake, Vaniman, Bish, Chipera, Morrison and Downs2014). In addition to the Fe3+ doublets, a minor Fe2+ doublet was also recognized, but the area ratio of the doublet was <8% (Tables 3 and 4).

Fig. 8. Representative Mössbauer spectra: a DH1-7 measured at room temperature; b DH1-4 measured at room temperature; c DH4-9 measured at room temperature; d DH1-7 measured at 77 K; e DH1-4 measured at 77 K; f DH4-9 measured at 77 K
Table 3 Computed Mössbauer parameters measured at room temperature
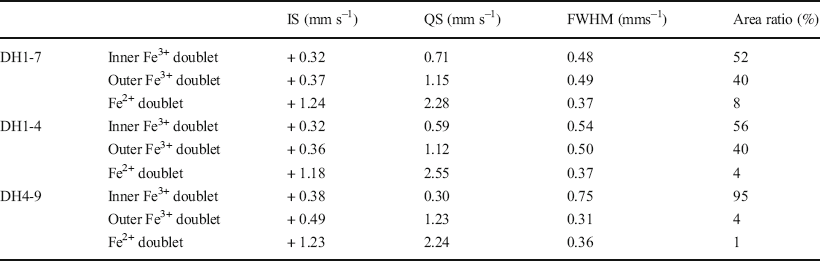
Table 4 Computed Mössbauer parameters measured at 77 K
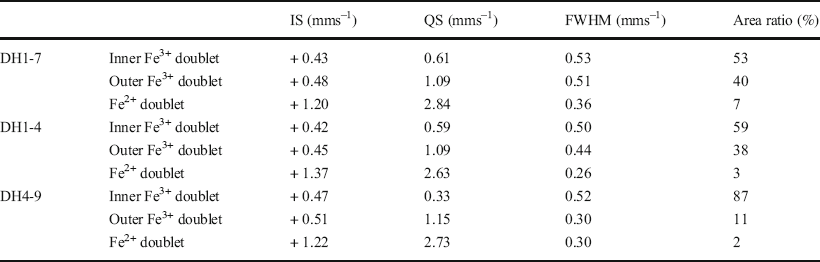
The Mössbauer spectra of the nontronite-like mineral differed from that of F-M-S-H and exhibited a predominant Fe3+ inner-doublet (Fig. 8 and Tables 3 and 4). The IS and QS values of the Fe3+ inner-doublet at room temperature (Table 3) displayed similar values to that of Fe3+ in the octahedral site of the TOT layer of nontronite, which exhibited an IS of 0.38 mms–1 and a QS of 0.25 mms–1 at room temperature (Johnston & Cardile, Reference Johnston and Cardile1985). In addition, minor Fe3+ outer-doublets were also recognized. The IS and QS values for the outer-doublet at room temperature (Table 3) was similar to that of Fe3+ in the octahedral site of the TOT layer of chlorite, which had an IS of 0.41 mms–1 and a QS of 1.03 mms–1 (Zazzi et al., Reference Zazzi, Hirsch, Leonova, Kaikkonen, Grins, Annersten and Edén2006). The outer-doublet also exhibited similar values to the interlayer Fe3+ ions of nontronite, which recorded an IS of 0.48 mms–1 and a QS of 1.94 mms–1 at room temperature (Johnston & Cardile, Reference Johnston and Cardile1985). An Fe2+ doublet was barely detected, and the area ratio of the doublet was <2% (Tables 3 and 4).
FTIR Spectra
The FTIR spectra for the OH-stretching vibration zones of both F-M-S-H and the nontronite-like mineral exhibited bands at 3676–3684 cm–1 (Fig. 9a), and were attributed to Mg3–OH stretching vibrations in the octahedral sheet (Grauby et al., Reference Grauby, Petit, Decarreau and Baronnet1994; Madejová, Reference Madejová2003). The bands at 3538–3547 cm–1 may have arisen from Fe2 3+–OH vibrations (Grauby et al., Reference Grauby, Petit, Decarreau and Baronnet1994; Zviagina et al., Reference Zviagina, McCarty, Środoń and Drits2004). Because the bands at 3538–3547 cm–1 were broad, the bands from Fe3+–OH–Mg vibrations at 3630 cm–1 can be added to these broad bands (Grauby et al., Reference Grauby, Petit, Decarreau and Baronnet1994). The bands at 3412–3414 cm–1 were due to adsorbed water (Brindley et al., Reference Brindley, Bish and Wan1979). Indeed, bands corresponding to water adsorbed by smectites were also observed previously even after heating pellets before the analysis (Zviagina et al., Reference Zviagina, McCarty, Środoń and Drits2004).

Fig. 9. Representative FTIR spectra of DH1-7, DH1-4, and DH4-9
The FTIR spectra of all samples exhibited strong bands at 1008–1020 cm–1 (Fig. 9b). These were attributed to Si–O stretching adsorption, which can be observed in various silicates (Farmer, Reference Farmer1958; Petit et al., Reference Petit, Decarreau, Gates, Andrieux and Grauby2015). The positions of the Si–O bands are known to shift toward 950 cm–1 as the amount of Fe3+ in the tetrahedral sheets increases (Baron et al., Reference Baron, Petit, Tertre and Decarreau2016). Thus, the positions at 1008–1020 cm–1 suggest that the F-M-S-H and nontronite-like mineral had little or no tetrahedral Fe3+ (Baron et al., Reference Baron, Petit, Tertre and Decarreau2016; Petit et al., Reference Petit, Decarreau, Gates, Andrieux and Grauby2015).
The FTIR spectra of all samples contained bands in the range between 440 and 447 cm–1 (Fig. 9b). These bands occurred in similar positions to in-plane Mg–O vibrations from serpentine (Yariv & Heller-Kallai, Reference Yariv and Heller-Kallai1975), and were also recognized in the IR spectra of saponite (Madejová et al., Reference Madejová, Balan and Petit2011). The band around 670 cm–1 appeared in the F-M-S-H samples at DH1-7, and the associated band shoulders were recognized in the F-M-S-H sample at DH1-4 and in the nontronite-like mineral at DH4-9 (Fig. 9b). They were also recognized in several Mg-rich smectites (Benhammou et al., Reference Benhammou, Tanouti, Nibou, Yaacoubi and Bonnet2009; Grauby et al., Reference Grauby, Petit, Decarreau and Baronnet1994; Shimoda, Reference Shimoda1971), and attributed to Mg3–OH vibrations (Grauby et al., Reference Grauby, Petit, Decarreau and Baronnet1994).
Small bands in the range 613–616 cm–1 were observed in all samples (Fig. 9b), but were difficult to assign; the bands occurred in similar positions to Si–O–Al bonds of tetrahedral Si–O and octahedral Al–O, however, which were observed for synthetic dioctahedral smectite (Petit et al., Reference Petit, Decarreau, Gates, Andrieux and Grauby2015). Thus, the small bands are probably derived from small amounts of Al in the octahedral sheets of F-M-S-H and the nontronite-like mineral. A small band at 857 cm–1 appeared only in the F-M-S-H samples at DH1-4 (Fig. 9b), but the attribution of the band is still debated (Petit et al., Reference Petit, Decarreau, Gates, Andrieux and Grauby2015).
The FTIR spectra of the nontronite-like mineral exhibited bands at 820 cm–1, while those of F-M-S-H showed only small bands at 816–820 cm–1 (Fig. 9b). These bands were related to Fe2 3+–OH vibrations from nontronite (Baron et al., Reference Baron, Petit, Tertre and Decarreau2016; Decarreau et al., Reference Decarreau, Petit, Martin, Farges, Vieillard and Joussein2008; Grauby et al., Reference Grauby, Petit, Decarreau and Baronnet1994). Unlike F-M-S-H , the nontronite-like minerals exhibited bands at 764 and 493 cm–1 (Figure 9b). The band at 764 cm–1 was attributed to Fe3+–Mg–OH vibrations, which were also observed in Fe3+-rich montmorillonite (Petit et al., Reference Petit, Caillaud, Righi, Madejová, Elsass and Köster2002). The band at 493 cm–1 was assigned to the change in Si–O–Fe3+ vibrations, which were also recognized in nontronite (Baron et al., Reference Baron, Petit, Tertre and Decarreau2016).
XRD Analysis and Chemical Composition after Acid or Alkylammonium Treatment of DH1-7
The expansion properties induced through the EG treatment of F-M-S-H (DH1-7) were unchanged regardless of the acid treatment (Figs 4a and 10). Nevertheless, peak shifts occurred in acid-treated F-M-S-H after heating. Specifically, without acid treatment practically no change in its peak positions was observed after heating at 250°C (Fig. 2a; Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020), but after acid treatment a small peak at ~10 Å was observed (Fig. 10). The chemical compositions of the acid-treated F-M-S-H in DH1-7 were primarily composed of Fe, Mg, Si, and Al ions. The (Fe+Mg)/(Si+Al) ratio and the (Fe+Mg+Al)/Si ratio decreased, while the Fe/Mg ratio did not change significantly after the acid treatment (Fig. 11a, b, and c). Moreover, Ca ions were not detected after acid treatment (Fig. 11d).

Fig. 10. XRD profile of acid-treated DH1-7 and that after EG treatment or heating at 250°C; Srp: serpentine

Fig. 11. Chemical composition of the samples before and after acid treatments or alkylammonium treatment; results are expressed as box-and-whisker plots. The solid line in the middle of the box represents the median values, whereas the ends of the boxes display the locations of the first and third quartiles (Q1 and Q3). The ends of the whiskers extend to the lowest and highest observations inside the region defined by Q1 – 1.5 (Q3–Q1) and Q3 + 1.5 (Q3–Q1). Values that fall beyond the whiskers are not plotted
When the alkylammonium treatment was conducted for the F-M-S-H in DH1-7, the 001 peak of F-M-S-H shifted from 14.6 to 21.1 Å (Fig. 12). The (Fe+Mg)/(Si+Al) ratio, the (Fe+Mg+Al)/Si ratio, and the Fe/Mg ratio of the F-M-S-H in DH1-7 did not change significantly after the alkylammonium treatment (Fig. 11a, b, and c). In addition, Ca ions were not detected after alkylammonium treatment (Fig. 11d).

Fig. 12. XRD profile of DH1-7 before and after alkylammonium treatment of oriented samples; Srp: serpentine
Discussion
F-M-S-H
Imperfect interlayer hydroxide sheets
F-M-S-H displayed an XRD peak at 14 Å (Fig. 4a and c). However, the expandability after EG treatment and the dehydration behavior of F-M-S-H showed distinct differences from chlorite and smectite (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). The unusual peak shift due to EG treatments and heating was similar to that of previously reported swelling chlorite and hydroxy-interlayered smectites (HIS). Swelling chlorite and HIS exhibited less expandability during EG treatment than smectite (swelling chlorite: Shimoda, Reference Shimoda1974; HIS: Meunier, Reference Meunier2007), although in certain instances, swelling chlorite showed a similar expandability to smectite (Bain & Russell, Reference Bain and Russell1981; Stephen & MacEwan, Reference Stephen and MacEwan1950). In addition, swelling chlorite and HIS also exhibited a different dehydration peak shift compared to smectite (Bain & Russell, Reference Bain and Russell1981; Meunier, Reference Meunier2007; Stephen & MacEwan, Reference Stephen and MacEwan1950). Bain and Russell (Reference Bain and Russell1981) showed that this XRD behavior of swelling chlorite is due to the interlayer hydroxide sheet being imperfect and present in small amounts i.e. enough to allow organic molecules in the interlayer to expand the structure, but enough to prevent structural shrinkage after heating. Therefore, the similarities between F-M-S-H and swelling chlorite or HIS imply that F-M-S-H contains imperfect interlayer hydroxide sheets between TOT layers.
According to the IR and the Mössbauer spectra, the (Fe+Mg)/(Si+Al) or (Fe+Mg+Al)/Si ratio indicates the ratio of octahedral to tetrahedral cations in the F-M-S-H, although the coordination of Al ions remains uncertain owing to its small amount. The decrease in the (Fe+Mg)/(Si+Al) and (Fe+Mg+Al)/Si ratios of F-M-S-H at DH1-7 caused by the acid treatment (Fig. 11a and b) implied that the number of octahedral cations decreased in comparison to those in tetrahedral coordination. In addition, as the peak at 10 Å appeared on the XRD profile during the heating of acid-treated F-M-S-H (Fig. 10), the acid treatment dissolved preferentially the imperfect interlayer hydroxide sheets, subsequently causing structural shrinkage of the TOT layers during heating. Indeed, a previous study reported that hydroxide sheets between the TOT layers were partially dissolved when swelling chlorite was immersed in acid (Rich & Bonnet, Reference Rich and Bonnet1975). In summary, supported by the results of characterization after acid treatment, F-M-S-H has Fe- and Mg-bearing imperfect interlayer hydroxide sheets between TOT layers.
Adsorbed cations and charges
The peak shift of F-M-S-H at DH1-7 induced by alkylammonium treatment indicated that F-M-S-H expanded due to the intercalation of alkylammonium ions (Fig. 12). As the only change observed in the chemical compositions after alkylammonium treatment was a decrease in Ca ions (Fig. 11), the Ca ions were probably adsorbed initially into the F-M-S-H interlayer and exchanged with alkylammonium ions during the treatment.
Although alkylammonium treatments have been used commonly to measure the layer charge of smectites and vermiculites (Lagaly & Weiss, Reference Lagaly, Weiss and Heller1969; Olis et al., Reference Olis, Malla and Douglas1990), the presence of interlayer hydroxide sheets in F-M-S-H prevented the determination of layer charge. Thus, the maximum layer charge derived from isomorphous substitution, which was obtained by assuming that all Al ions were tetrahedral, was calculated from the median value of the Al/(Si+Al) ratios (Table 2). The maximum negative charge due to the isomorphous substitution of F-M-S-H at DH1-7 was 0.44 per O10(OH)2 in TOT layers. On the other hand, the positive charge due to adsorbed Ca ions at DH1-7 was 0.83 per O10(OH)2 in TOT layers, which was calculated from the Ca/(Si+Al) ratios (Table 2). The charge imbalances could be derived from the defect of cations in octahedral sheets and from anomalously high variable charges. If assuming the charge imbalances can be explained by only the additional considerations surrounding the defect of divalent cations in octahedral sheets, such as stevensite, the number of cations in octahedral sheets could be 2.81 per O10(OH)2 in TOT layers. On the other hand, it seems more plausible to consider the anomalously high variable charges derived from smaller particle size or from less crystallinity than in optimum TOT layers, based on the fact that F-M-S-H exhibited relatively broader peaks and lower intensities on the XRD profile of the <2 μm fractions (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020; Fig. 4a and c). According to the median values of the Al/(Si+Al) ratios and the Ca/(Si+Al) ratios of other F-M-S-H (Table 2), the negative charge derived from the defect of octahedral cations and from anomalously high variable charge should be considered for all F-M-S-H.
Unusual trioctahedral clay with Fe3+ as a major element
The major d 060 values of F-M-S-H suggested a similarity to trioctahedral phyllosilicates (e.g. Fig. 4b and d). This is consistent with the fact that the FTIR results of F-M-S-H were indicative primarily of trioctahedral characteristics (Fig. 9b), although some dioctahedral characteristics were also present among the FTIR spectra. Nevertheless, the Mössbauer spectra showed that F-M-S-H is rich in ferric iron (Tables 3 and 4), suggesting a dioctahedral character based on the common Fe3+-rich phyllosilicates. The F-M-S-H samples thus appear to have characteristics different from common phyllosilicates in that they have a trioctahedral character even though some Fe3+ is present.
Previous studies reported a rapid oxidation of ferrous iron-bearing smectite in glassy rhyolitic tuffs in Japan (Kohyama et al., Reference Kohyama, Shimoda and Sudo1973). Similarly, the oxidation of ferrous iron-bearing smectite was recognized in a gabbro saprolite in Canada (Kodama et al., Reference Kodama, De Kimpe and Dejou1988) as well as in basaltic rocks in the USA (Treiman et al., Reference Treiman, Morris, Agresti, Graff, Achilles, Rampe, Bristow, Ming, Blake, Vaniman, Bish, Chipera, Morrison and Downs2014). These clays displayed a d 060 value similar to trioctahedral phyllosilicates although they contained certain amounts of Fe3+. The FTIR spectra of these clays displayed bands in similar positions to those of Mg-rich trioctahedral phyllosilicates (Kodama et al., Reference Kodama, De Kimpe and Dejou1988; Kohyama et al., Reference Kohyama, Shimoda and Sudo1973). In addition, the Mössbauer spectra revealed high QS values (Treiman et al., Reference Treiman, Morris, Agresti, Graff, Achilles, Rampe, Bristow, Ming, Blake, Vaniman, Bish, Chipera, Morrison and Downs2014), which was also the case for the F-M-S-H. The increase in QS values may derive from the distortion of crystallographic sites (Dyar et al., Reference Dyar, Agresti, Schaefer, Grant and Sklute2006) or from low crystallinity (Baron et al., Reference Baron, Petit, Pentrák, Decarreau and Stucki2017). Because Kohyama et al. (Reference Kohyama, Shimoda and Sudo1973) and Treiman et al. (Reference Treiman, Morris, Agresti, Graff, Achilles, Rampe, Bristow, Ming, Blake, Vaniman, Bish, Chipera, Morrison and Downs2014) suggested previously that the lattice in oxidized ferrous iron-bearing smectite becomes distorted or contracted, the more plausible assumption here is that the high QS values exhibited were due mainly to distortion through oxidation. Thus, F-M-S-H exhibited similar characteristics to those of the oxidized ferrous iron-bearing smectite from various regions, indicating that F-M-S-H was initially ferrous and then was oxidized.
Variation with sampling depths and formation pathway
The decreases in the (Fe+Mg)/(Si+Al) and (Fe+Mg+Al)/Si ratios of F-M-S-H as sampling depths increased suggested that the number of octahedral cations decreased relative to those in tetrahedral coordination (Fig. 7a and b). In addition, the peaks observed at ~10 Å during heating, which were indicative of a domain without interlayer hydroxide sheets, appeared with increasing sampling depths (Fig. 2; Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). The implication is, therefore, that the number of interlayer hydroxide sheets of F-M-S-H decreased gradually with increasing sampling depth, and formed smectitic domains (i.e. domains without interlayer hydroxide sheets; Fig. 13).

Fig. 13. Schematic of the F-M-S-H structural characteristics with different sampling depths
One possibility that led to a relative decrease in interlayer hydroxide sheets with sampling depth could be that changes occurred in the mineral assemblage of the remaining primary minerals. Shimbashi et al. (Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020) suggested that Mg-rich olivine was present in the initial sediments and dissolved through an interaction with alkaline seepage. After olivine had dissolved, the primary minerals which remained (e.g. pyroxene, amphibole) exhibited smaller (Mg+Fe)/Si ratios. If this change in the mineral assemblage of primary minerals leads to the gradual alteration of F-M-S-H, one would expect that F-M-S-H will gradually alter to show smaller (Fe+Mg)/Si ratios, i.e. the relative decrease in interlayer hydroxide sheets.
A previous study at the focal site reported a positive linear correlation between sampling depths and the depositional ages of the sediments (Table 1; Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). If the sediments interacted with alkaline seepage after deposition, it is considered that the deeper sediments were interacting with alkaline seepage for a longer period. Therefore, although the cause of the change in the expandability, the Fe/Mg ratios, and the Al/(Si+Al) ratios of F-M-S-H remains uncertain (Figs 5 and 7), the mineralogical changes exhibited in association with increasing sampling depths were also probably caused by the varied interaction periods between each sediment at different sampling depths and alkaline seepage. In addition, worth noting is that changes in the characteristics of F-M-S-H according to sampling depth should have already been observed prior to the oxidation event. This is because changes in the characteristics of F-M-S-H in its ferrous form (F2+–M–S–H) at deeper depths should have already been initiated due to its interaction between the sediments and alkaline seepage, concurrently with the formation of F2+–M–S–H at shallower depths under relatively reducing conditions. Thus, F-M-S-H might have been oxidized, for example, after sample collection.
Nontronite-like Mineral
Structural and chemical characteristics
The nontronite-like mineral displayed an XRD peak at 14 Å and exhibited the same expandability after EG treatment as ideal smectite (d 001 at 17 Å) (Fig. 4e and Table 1). The results of the major d 060 on the XRD analysis were suggestive of Fe3+-rich dioctahedral sheets (i.e. nontronite) (Fig. 4f). The predominant inner-doublet of the nontronite-like mineral in the Mössbauer spectra suggested that Fe3+ ions were present in octahedral coordination in an environment similar to that of Fe3+ at the octahedral site of the ideal nontronite. Moreover, the chemical composition of the nontronite-like mineral showed that both Fe and Mg were predominant (Fig. 6a). Although Mg3–OH vibrations in the FTIR spectra could be derived partly from traces of serpentine in the <2 μm fraction (Fig. 4e), these bands were also observed in Fe3+- and Mg-bearing smectites (Grauby et al., Reference Grauby, Petit, Decarreau and Baronnet1994). Thus, all of the data above indicated that the 14 Å phase in DH4-9 can be assigned to nontronite containing certain amounts of Mg in the octahedral sheets.
In contrast, the peak that indicated no structural shrinkage upon heating at 250°C suggested the presence of a phase different from ideal nontronite (Fig. 3; Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). Considering the same for F-M-S-H, this indicated that an imperfect interlayer hydroxide sheet was present partly between layers of nontronite – the 14 Å phase; this is referred to as the nontronite-like mineral, consisting of nontronite, partly including the interlayer hydroxide sheet.
Formation Pathway
Distinct differences in chemical compositions and Fe sites between the F-M-S-H and the nontronite-like mineral were recognized (Figs 6b and 8). Shimbashi et al. (Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020) suggested that the F-M-S-H was formed as the result of the interaction between sediments and alkaline seepage without the involvement of seawater, whereas the nontronite-like mineral formed from the interaction with seawater infiltration. Therefore, the distinct differences between F-M-S-H and the nontronite-like mineral can be attributed to these phases being formed under different chemical conditions.
Considering that nontronite can form under various redox conditions from either Fe2+ or Fe3+ starting gels (Petit et al., Reference Petit, Baron and Decarreau2017), several pathways for nontronite formation are possible. For example, Decarreau and Bonnin (Reference Decarreau and Bonnin1986) and Decarreau et al. (Reference Decarreau, Petit, Martin, Farges, Vieillard and Joussein2008) reported that nontronite formed through an initial Fe2+-bearing precipitate and subsequent oxidation (Pathway I). In addition, Decarreau et al. (Reference Decarreau, Bonnin, Badaut-Trauth, Couty and Kaiser1987) reported that the synthesis of smectite at low temperatures from solutions that contained Fe3+ ions without Fe2+ or Mg ions was also possible (Pathway II), although the crystal growth of a ferric smectite under those conditions was slow. Gainey et al. (Reference Gainey, Hausrath, Adcock, Tschauner, Hurowitz, Ehlmann, Xiao and Bartlett2017) reported that dioctahedral Fe-rich clay minerals, such as nontronite, can be formed rapidly from aqueous Fe3+ in the presence of at least small amounts of aqueous Mg (Pathway III).
Regarding the formation of the nontronite-like mineral at the study site, the likelihood that it formed through the Pathway II process was low; this is because a certain amount of Mg was recognized in its chemical composition (Fig. 6a). Moreover, if the nontronite-like mineral was formed through Pathway I, an ejection of ferric iron from the octahedral sheets with oxidation and the formation of iron oxides either inside or outside the interlayer would be expected to occur to form a dioctahedral sheet of nontronite-like mineral. However, the formation of interlayer hydroxide sheets by the ejected ferric iron was not plausible in the present study because the chemical composition of the nontronite-like mineral exhibited a similarity to nontronite (Fig. 6b). Moreover, the formation of iron oxides outside the nontronite-like mineral were not observed during the current analysis, so formation through Pathway I was not likely. From the above consideration, the nontronite-like mineral possibly was precipitated directly in the presence of aqueous Fe3+ and Mg ions (i.e. Pathway III).
In contrast to F-M-S-H, the nontronite-like mineral exhibited no significant changes in expandability and chemical composition with varying sampling depths (Figs 5 and 7). This indicates no correlation between the structural and chemical characteristics of the nontronite-like mineral and the period of interaction between the sediments and alkaline seepage. The depositional environment of the sediments probably changed from seawater to brackish and then to freshwater, and the nontronite-like mineral was thought to be formed during a period of seawater infiltration (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). This indicates that the nontronite-like mineral has likely been stable under present-day alkaline conditions for thousands of years (Table 1), after the cessation of seawater infiltration.
Conclusions
In order to predict the potential alteration of the constituent minerals in radioactive-waste disposal facilities, understanding secondary mineral species under natural alkaline conditions is important. A previous study reported uncommon Fe- and Mg-bearing clays, referred to as F-M-S-H and nontronite-like minerals, which were formed under natural alkaline conditions (Shimbashi et al., Reference Shimbashi, Yokoyama, Watanabe, Sato, Otake, Kikuchi, Yamakawa and Fujii2020). In the current study, the structural and chemical characteristics and formation pathways of these clays were investigated.
F-M-S-H comprised TOT layers, imperfect interlayer hydroxide sheets, and interlayer Ca ions. F-M-S-H was previously of a ferrous form, and systematic changes, such as the gradual decrease of the interlayer hydroxide sheets to create smectitic domains, occurred during the interaction between each sediment at different sampling depths and alkaline seepage. Furthermore, F-M-S-H likely oxidized after sample collection.
The nontronite-like mineral comprised nontronite and partly included an interlayer hydroxide sheet. The nontronite-like mineral was formed under different chemical conditions from F-M-S-H, likely having been precipitated in the presence of aqueous Fe3+ and Mg ions during a period of seawater infiltration. Mineralogical changes did not occur after the cessation of seawater infiltration, at least among the timescales observed at the study site, i.e. they were likely stable under present-alkaline conditions for thousands of years.
Acknowledgments
This study was carried out as part of the Natural Analogue project within the “Advanced technology development for geological disposal of TRU waste” program commissioned by the Agency for Natural Resources and Energy in the Ministry of Economy, Trade and Industry of Japan. The authors thank Dr Fujii Naoki from Radioactive Waste Management Funding and Research Center for the principal management of the project. They also thank Dr Minoru Yamakawa from the Radioactive Waste Management Funding and Research Center, Mr Masanobu Nishimura and Mr Yukinobu Kimura of the Obayashi Corporation, Dr W. Russell Alexander from Bedrock Geosciences, and Professor Carlo Arcilla at the University of the Philippines for the field survey. Dr Yasutaka Watanabe and Dr Daisuke Minato from the Central Research Institute of Electric Power Industry are acknowledged for their help and valuable comments for performing research at this site. The authors thank the two anonymous reviewers for considerably improving the manuscript.
Funding
This work was funded by JSPS KAKENHI (grant number JP19H00878).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable
Declarations
Conflicts of interest
The authors declare that they have no conflict of interest.



















