Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Martin, F.
Petit, S.
Decarreau, A.
Grauby, O.
Hazemann, J.L.
and
Noack, Y.
1992.
Experimental study of SiGe tetrahedral solid solution in NiCoMg talcs.
Thin Solid Films,
Vol. 222,
Issue. 1-2,
p.
189.
Creach, M.
and
Magonthier, M.C.
1992.
Retention of radionuclides in poorly organized phases in the near field of a HLW nuclear disposal facility.
Applied Clay Science,
Vol. 7,
Issue. 1-3,
p.
59.
Burns, Roger G
1993.
Rates and mechanisms of chemical weathering of ferromagnesian silicate minerals on Mars.
Geochimica et Cosmochimica Acta,
Vol. 57,
Issue. 19,
p.
4555.
Köhler, Birgit
Singer, Arieh
and
Stoffers, Peter
1994.
Biogenic Nontronite from Marine White Smoker Chimneys.
Clays and Clay Minerals,
Vol. 42,
Issue. 6,
p.
689.
Charlet, Laurent
and
Manceau, Alain
1994.
Evidence for the neoformation of clays upon sorption of Co(II) and Ni(II) on silicates.
Geochimica et Cosmochimica Acta,
Vol. 58,
Issue. 11,
p.
2577.
Vidal, Olivier
Magonthier, Marie-Claude
Joanny, Véronique
and
Creach, Monique
1995.
Partitioning of La between solid and solution during the ageing of SiAlFeLaCa gels under simulated near-field conditions of nuclear waste disposal.
Applied Geochemistry,
Vol. 10,
Issue. 3,
p.
269.
Manceau, A.
Ildefonse, Ph.
Hazemann, J. -L.
Flank, A-M.
and
Gallup, D.
1995.
Crystal Chemistry of Hydrous Iron Silicate Scale Deposits at the Salton Sea Geothermal Field.
Clays and Clay Minerals,
Vol. 43,
Issue. 3,
p.
304.
Ildefonse, Ph.
Calas, G.
Flank, A.M.
and
Lagarde, P.
1995.
Low Z elements (Mg, Al, and Si) K-edge X-ray absorption spectroscopy in minerals and disordered systems.
Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms,
Vol. 97,
Issue. 1-4,
p.
172.
Münch, Philippe
Duplay, Joëlle
and
Cochemé, Jean-Jacques
1996.
Alteration of Silicic Vitric Tuffs Interbedded in Volcaniclastic Deposits of the Southern Basin and Range Province, Mexico: Evidences for Hydrothermal Reactions.
Clays and Clay Minerals,
Vol. 44,
Issue. 1,
p.
49.
Lajarige, Christelle
Petit, Sabine
Augas, Claude
and
Decarreau, Alain
1998.
Stabilisation d'ions Fe2+ dans le réseau de smectites ferrifères de synthèse.
Comptes Rendus de l'Académie des Sciences - Series IIA - Earth and Planetary Science,
Vol. 327,
Issue. 12,
p.
789.
Ece, Ö. Işik
Çoban, Fazli
Güngör, Nurfer
and
Suner, Fikret
1999.
Clay Mineralogy and Occurrence of Ferrian Smectites Between Serpentinite Saprolites and Basalts in Biga Peninsula, Northwest Turkey.
Clays and Clay Minerals,
Vol. 47,
Issue. 3,
p.
241.
Kloprogge, J. Theo
Komarneni, Sridhar
and
Amonette, James E.
1999.
Synthesis of Smectite Clay Minerals: A Critical Review.
Clays and Clay Minerals,
Vol. 47,
Issue. 5,
p.
529.
Nagase, Takako
Iwasaki, Takeshi
Ebina, Takeo
Hayashi, Hiromichi
Onodera, Yoshio
and
Dutta, Niranjan Chandra
1999.
Hydrothermal Synthesis of Fe-montmorillonite in Si-Fe-Mg System.
Chemistry Letters,
Vol. 28,
Issue. 4,
p.
303.
Haese, Ralf R.
2000.
Marine Geochemistry.
p.
233.
Ueshima, Masato
and
Tazaki, Kazue
2001.
Possible Role of Microbial Polysaccharides in Nontronite Formation.
Clays and Clay Minerals,
Vol. 49,
Issue. 4,
p.
292.
Taitel-Goldman, Nurit
and
Singer, Arieh
2001.
High-Resolution Transmission Electron Microscopy Study of Newly Formed Sediments in the Atlantis II Deep, Red Sea.
Clays and Clay Minerals,
Vol. 49,
Issue. 2,
p.
174.
Taitel-Goldman, N.
and
Singer, A.
2002.
Metastable Si-Fe phases in hydrothermal sediments of Atlantis II Deep, Red Sea.
Clay Minerals,
Vol. 37,
Issue. 2,
p.
235.
Villemure, Gilles
2002.
Electrochemical Properties of Clays.
p.
149.
Pokrovski, Gleb S
Schott, Jacques
Hazemann, Jean-Louis
Farges, François
and
Pokrovsky, Oleg S
2002.
An X-ray absorption fine structure and nuclear magnetic resonance spectroscopy study of gallium–silica complexes in aqueous solution.
Geochimica et Cosmochimica Acta,
Vol. 66,
Issue. 24,
p.
4203.
Pokrovski, Gleb S
Schott, Jacques
Farges, François
and
Hazemann, Jean-Louis
2003.
Iron (III)-silica interactions in aqueous solution: insights from X-ray absorption fine structure spectroscopy.
Geochimica et Cosmochimica Acta,
Vol. 67,
Issue. 19,
p.
3559.
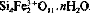 , were aged in suspension at 75°, 100° and 150°C and the structural evolution of solids with time studied by XRD, TEM, and IR, Mössbauer and EXAFS spectroscopy. The initial Si-Fe coprecipitate was found to be amorphous but showed local order similar to that of a smectite layer. At 75°C only a weak structural evolution of the silico-ferric product towards a smectite-like structure was observed. Experiments performed at 100° and 150°C led to synthesis of a ferric smectite with structural formula
, were aged in suspension at 75°, 100° and 150°C and the structural evolution of solids with time studied by XRD, TEM, and IR, Mössbauer and EXAFS spectroscopy. The initial Si-Fe coprecipitate was found to be amorphous but showed local order similar to that of a smectite layer. At 75°C only a weak structural evolution of the silico-ferric product towards a smectite-like structure was observed. Experiments performed at 100° and 150°C led to synthesis of a ferric smectite with structural formula 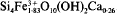 . During syntheses a highly soluble silico-ferric complex appeared; the Si/Fe atomic ratio of this complex was 3, and the apparent concentration of Fe3+ in solution reached 27 mm/l. These syntheses prove that the crystallization of a dioctahedral smectite, containing only Fe3+ atoms in the octahedral sheet, is possible under strictly oxidizing conditions. However, crystal growth of a ferric smectite under these conditions is slow and only syntheses carried out at sufficiently high temperatures give convincing results.
. During syntheses a highly soluble silico-ferric complex appeared; the Si/Fe atomic ratio of this complex was 3, and the apparent concentration of Fe3+ in solution reached 27 mm/l. These syntheses prove that the crystallization of a dioctahedral smectite, containing only Fe3+ atoms in the octahedral sheet, is possible under strictly oxidizing conditions. However, crystal growth of a ferric smectite under these conditions is slow and only syntheses carried out at sufficiently high temperatures give convincing results.



