Growth failure is highly prevalent among infants with CHD.Reference Varan, Tokel and Yilmaz 1 – Reference Hehir, Easley and Byrnes 3 Children with the most complex CHD have a particularly high prevalence of growth failure. One study of children with hypoplastic left heart syndrome found that the median World Health Organization weight-for-age z-score at the time of bi-directional Glenn operation was −2.0, meaning that 50% were moderately or severely malnourished.Reference Kelleher, Laussen, Teixeira-Pinto and Duggan 4 Growth failure is probably multifactorial in aetiology, including factors such as inadequate caloric intake, high metabolic demands, gastrointestinal physiology, and genetic and extracardiac abnormalities.
Growth failure has been associated with adverse clinical outcomes in children with CHD. Lower weight-for-age z-score at the time of bi-directional Glenn has been associated with longer hospital stay.Reference Anderson, Beekman and Border 5 In a case–control study of late death after repair for a variety of CHD, children who died demonstrated continued decline in weight-for-age z-score, whereas survivors maintained their weight.Reference Eskedal, Hagemo and Seem 6 However, even the survivors are at risk of poor growth; a study of postoperative feedings in CHD infants found median daily weight gain to be very low in nearly one-third of the infants discharged home at below their birth weight.Reference Natarajan, Reddy Anne and Aggarwal 7
Despite the high prevalence of malnutrition and associations with adverse clinical outcomes, there is currently little evidence guiding optimal nutritional management strategies for patients with CHD, especially those with severe CHD. A recent survey of 46 centres participating in the National Pediatric Cardiology Quality Improvement Collaborative confirmed wide variation in perioperative feeding and evaluation practices in centres caring for single-ventricle patients.Reference Slicker, Sables-Baus, Lambert, Peterson, Woodard and Ocampo 8 Among the centres, 65% performed preoperative feeding, with varied routes. In total, 67% of centres did not use vital sign thresholds to determine when to withhold feeding. In the postoperative setting, 46% of centres used an “internal guideline” and 33% used an “informal practice” to determine feeding readiness. In all, 40% of centres did not discharge patients home with feeding tubes. Among those who did, there was no consensus on post-discharge feeding tube modality.
In June, 2011, a Standardized Clinical Assessment and Management Plan (SCAMP®) was introduced at our institution to guide evaluation and treatment of growth failure in cardiac patients seen on the medical and surgical wards by identifying patients at risk and recommending appropriate consults and procedures. A SCAMP is a practical and flexible tool for narrowing practice variability while simultaneously permitting providers to exercise their clinical acumen and adapt treatment pathways to individual patients’ needs.Reference Rathod, Farias and Friedman 9 , Reference Farias, Jenkins and Lock 10 The SCAMP process guides management of patients meeting certain criteria for enrolment, although clinicians may divert their patients from the SCAMP at their discretion, and assigns team members to collect data to evaluate practice and iteratively amend decision support algorithms if necessary. To date, two iterations of the feeding and nutrition SCAMP have been implemented. The purpose of this study was to determine whether enrolment in the most recent version of the SCAMP resulted in growth benefits beyond discharge, and whether SCAMP-enrolled patients grew better than those patients who were not enrolled by clinician choice (diversions), as well as when compared with a group of historical control patients.
Materials and method
The first Feeding and Nutrition SCAMP (iteration I) was implemented from June, 2011 through June, 2012, after which it underwent extensive modification following interval data analysis based on multiple diversions and inadequate weight gain. The revised SCAMP (iteration II) was implemented in July, 2013, was used through June, 2014, and included patients seen in the medical ward alone. This was a retrospective cohort study of three groups: the most recent group of SCAMP-enrolled CHD patients (iteration II), one group of SCAMP iteration II-diverted patients, and one group of historical CHD controls (Table 1).
Table 1 Group inclusion/exclusion criteria
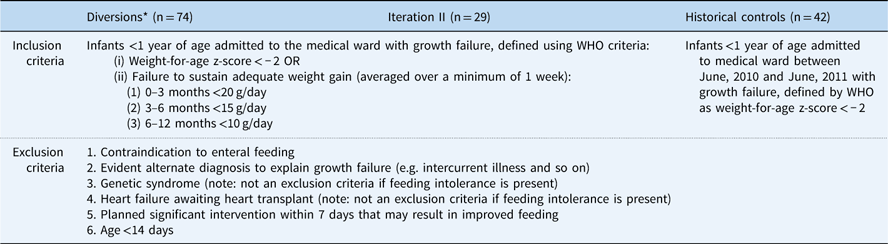
* Diverted patients were defined as patients from iteration II who met growth failure criteria but also met exclusion criteria or were otherwise diverted from enrolment at the discretion of the attending physician
Iteration II patients were defined as those who met Feeding and Nutrition SCAMP inclusion criteria.
Inclusion criteria for iteration II were infants <1 year of age who were admitted to the medical service, with growth failure defined as a World Health Organization weight-for-age z-score of <−2, or failure to sustain adequate weight gain averaged over a minimum of 1 week. Exclusion criteria for iteration II included contraindication to enteral feeding; evident alternate diagnosis to explain growth failure, such as intercurrent illness; genetic syndrome – not an exclusion criterion if feeding intolerance is present; heart failure awaiting heart transplant – not an exclusion criterion if feeding intolerance is present; planned significant intervention within 7 days that may result in improved feeding; or age<14 days.
Diverted patients were defined as patients from iteration II who met growth failure criteria but also met exclusion criteria or were otherwise diverted from enrolment at the discretion of the attending physician. As an integral part of the SCAMPs iterative process, diverted patients were analysed to explore whether inclusion criterion should be modified in future iterations. The reason for diversion was recorded and categorised into major groupings: planned intervention that could affect feeding, plan to admit the patient for less than 48 hours or expected transfer to the cardiology ICU, presence of intercurrent illness, other reasons given at the discretion of the attending, and unknown.
A historical control group was derived by identifying from a central data warehouse all patients who were seen on the cardiac medical floor with World Health Organization weight-for-age z-score<−2 from June, 2010 to June, 2011 before initiation of the SCAMP. Iteration II exclusion criteria were then applied to this group. The goal was to create a population that would have been eligible and considered for enrolment in the iteration II version of the SCAMP.
Control patients had admission weights and heights collected from a central data warehouse. The enrolment, admission, and most recent weights and heights were derived from a central data warehouse for patients in all groups. Additional variables were abstracted from the medical chart or were part of the SCAMP’s data collection efforts. The final data set included primary diagnosis; cardiac category, including heart muscle disease, heart rhythm disease, palliated CHD, repaired CHD, and unrepaired CHD; biventricular versus univentricular circulation; heterotaxy status; and prior surgical and catheter interventions in addition to other variables. For all groups, weights and heights were converted to z-scores via 2012 world population World Health Organization charts. Further, patients were only included in the analysis if they had follow-up information at least 6 months after SCAMP enrolment or diversion, or medical ward admission for controls.
The major parts of the decision support tool for SCAMP iteration II are shown in Figure 1. The attending on service was provided with a list of potentially eligible patients based on weight-for-age z-score criteria by a SCAMP data coordinator. Patients with poor weight gain were identified by the medical team on rounds. Eligible patients were further screened for exclusion criteria, and were enrolled at the discretion of the attending. Patients who met eligibility criteria but were not enrolled were considered to be diversions.
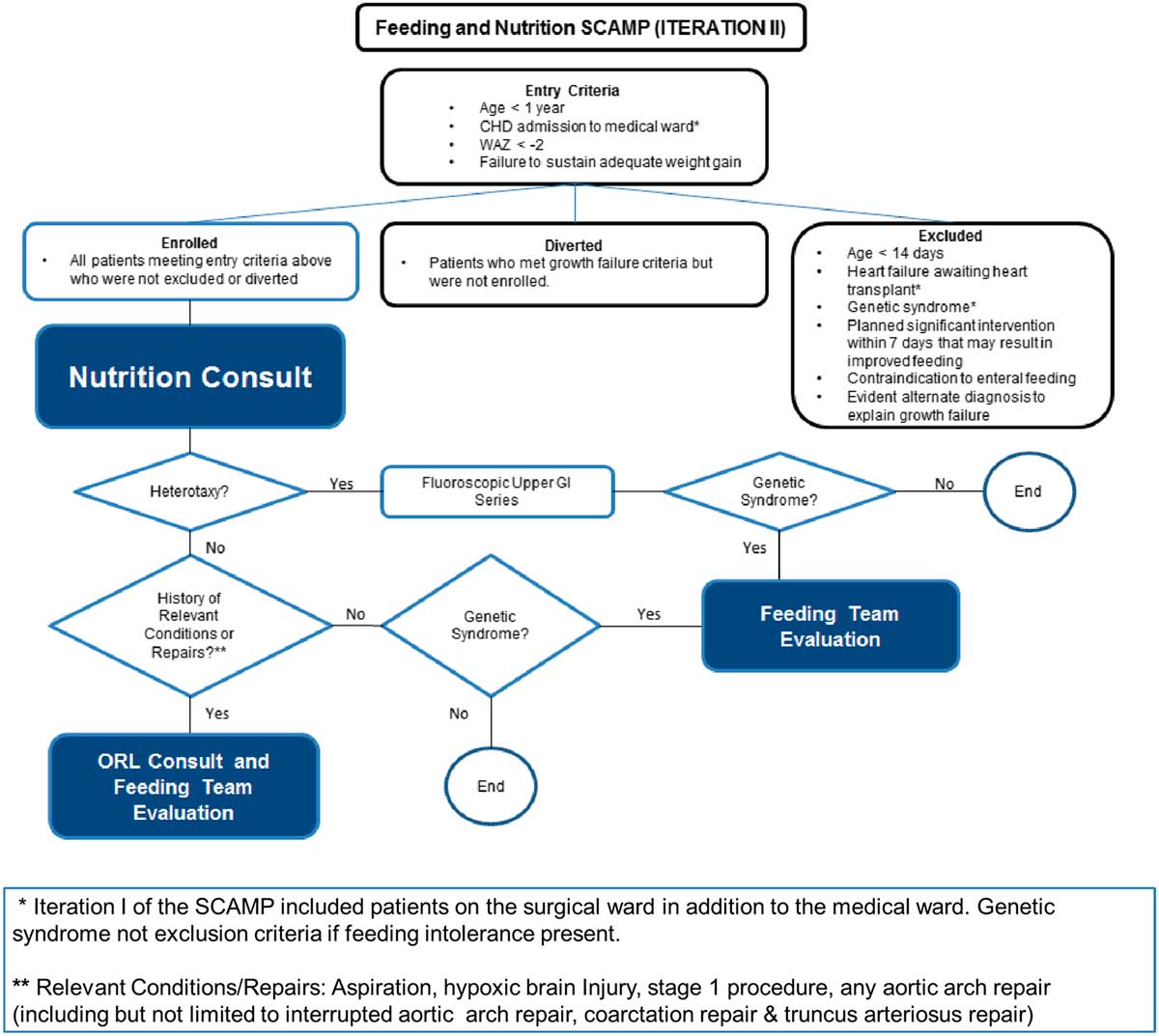
Figure 1 SCAMP algorithm for CHD infants with growth failure.
Enrolled patients had a comprehensive nutrition evaluation by a registered dietitian, who communicated daily with the care team and attended rounds if possible. Once weekly, a nutrition attending physician also communicated directly with the medical team, to discuss the patient’s nutritional plan. Patients received an otolaryngology consult for specific conditions such as aspiration, hypoxic brain injury, stage 1 procedure, or aortic arch repair or truncus arteriosus repair. Completion of data forms by clinical staff was facilitated by a SCAMP data coordinator.
Continuous variables are summarised with means, standard deviations, and 95% confidence intervals as noted or medians with 25th and 75th percentiles, and categorical variables are summarised as numbers and percentages. The primary outcome variable was change in weight-for-age z-score from baseline (WAZ1) to most recent follow-up (WAZ2), calculated as WAZ2−WAZ1 for each patient. The primary analysis compared the means of this outcome for SCAMP iteration II patients with historical controls using the two-sample t-test with Satterthwaite correction, which does not assume equal variances for the two groups, among patients with at least 6 months of follow-up. Medians were compared using the Wilcoxon rank-sum test. Secondary analyses compared change in weight-for-age z-score for iteration II to diverted patients, also using the two-sample t-test. Comparisons of categorical variables between groups was performed using Fisher’s exact test. Linear regression analysis was used to compare difference in mean change in weight-for-age z-score for iteration II patients versus controls, adjusting for status of having had a previous interventional catheterisation. Linear regression was also used to compare mean change in weight-for-age z-score for these two groups adjusting for baseline weight-for-age z-score. For the primary analysis and descriptive analyses, a significance level of 0.05 was used; for the secondary analyses, the significance level was set at 0.025 to account for multiple comparisons.
Results
Among 188 patients identified, 145 patients had at least a 6-month follow-up and were included in the analytical groups: iteration II, 29; diversions/exclusions, 74; controls, 42. Common reasons for diversion/exclusion from the SCAMP included planned intervention in the operating room or catheterisation lab (44%), planned admission for less than 48 hours (19%), expected transfer to the cardiology ICU (8%), presence of intercurrent illness upon admission (12%), undocumented reason (14%), or other uncategorised reason (3%). Among the analytical group, CHD diagnoses varied widely. Common diagnoses included hypoplastic left heart syndrome (13%), ventricular septal defects (13%), and tetralogy of Fallot (11%) (Table 2). Baseline characteristics other than weight of all groups were generally similar and are shown in Table 3, with statistical comparisons between iteration II and historical controls. Iteration II patients were younger than control patients at baseline, although not significantly so, with a median of 95 days (71, 156) versus 118 days (71, 200) (p=0.33). SCAMP enrolled patients had less median follow-up time from baseline to most recent weight measurement to achieve gains in weight-for-age z-score [647 days (398, 763) versus 1425.5 days (1217, 1579), p<0.001] compared with controls. Iteration II patients were also more likely to have had one or more catheterisations before enrolment (59 versus 33%, p=0.05).
Table 2 Frequency and percentage of primary cardiac diagnosis
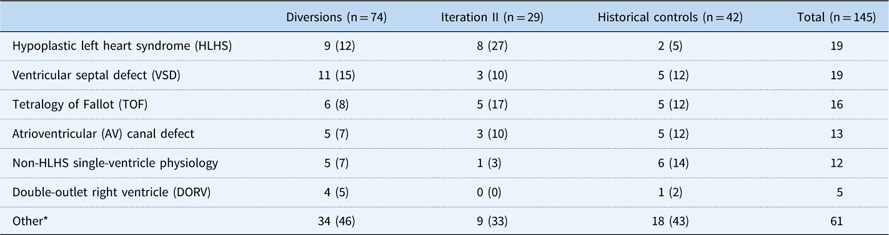
* Other diagnoses included the following: pulmonary vein stenosis, aortic coarctation, tricuspid atresia, aortic stenosis, tetralogy of Fallot with pulmonary atresia, transposition of the great arteries, pulmonary stenosis, total anomalous pulmonary venous return, pulmonary–arterial hypertension, tricuspid regurgitation, atrial septal defect, heart transplant, mitral regurgitation, mitral stenosis, patent ductus arteriosus, Shone’s complex, valvar regurgitation, valvar stenosis, and other uncategorised diagnoses.
Table 3 Patient baseline characteristics.
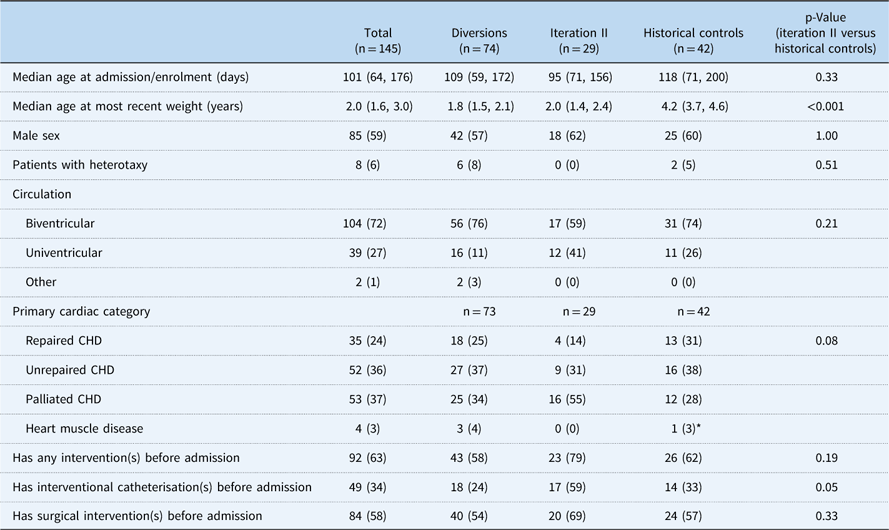
Data are presented as n (%) for categorical variables and median (25th and 75th percentiles) for continuous variables
* Not included in analysis
Baseline growth parameters for the four groups are shown in Table 4, with statistical comparisons performed between iteration II and controls. Patients enrolled in SCAMP iteration II, diverted patients, and historical controls showed improved weight-for-age z-score compared with baseline admission, most notably in the iteration II group (Fig 2). Compared with controls, iteration II patients had a lower mean weight-for-age z-score at baseline [−3.7 (−4.3, −3.0) versus −2.9 (−3.1, −2.6) (p=0.02)], and a greater change in weight-for-age z-score when measured at their most recent follow-up [2.7 (2.0, 3.4) versus 1.8 (1.5, 2.2) (p=0.03)]. Change in weight-for-age z-score was not significantly different between groups after adjusting for baseline weight-for-age z-score (p=0.61); iteration II patients started with lower z-scores at baseline and caught up with controls over the course of follow-up, displaying similar weight-for-age z-score at the most recent time point. When controlling for any previous catheterisations, the mean difference in weight-for-age z-score between groups decreased slightly from 0.9 to 0.7, with iteration II patients still higher than controls (p=0.06). In secondary analyses, iteration II had a greater weight-for-age z-score gain than the diverted group, 2.7 (2.0, 3.4) versus 1.4 (1.0, 1.7) (p<0.001).
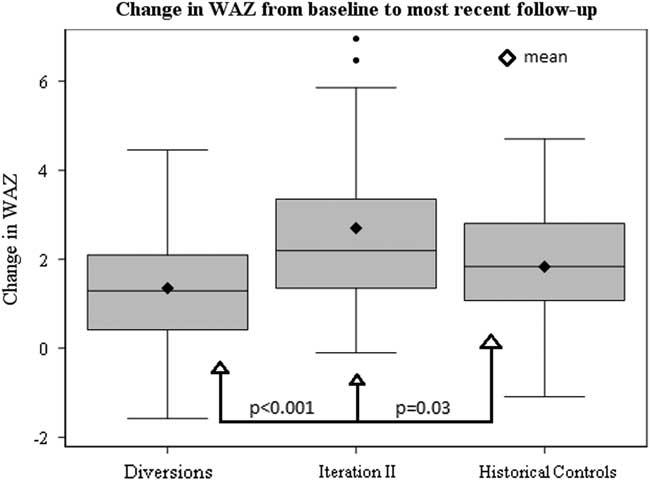
Figure 2 Box and whiskers plot depicting distributions in change in WAZ from baseline to most recent follow-up among SCAMP Iteration II patients, diverted patients and historical controls.
Table 4 Baseline, Standardized Clinical Assessment and Management Plan (SCAMP) exit, most recent, and change in World Health Organization (WHO) weight-for-age z-scores
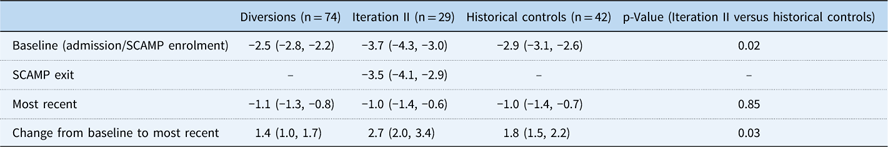
Baseline and most recent WHO weight-for-age z-scores are presented as mean and 95% CI. Changes in z-scores are present as positive mean change from baseline to most recent measurement and 95% CI.
Discussion
Our results demonstrate that the use of a SCAMP decision support tool, including a full nutrition assessment with nutrition goals, identification of key diagnoses that may affect feeding, and direct communication between a nutritionist and nutrition attending with the cardiology team, resulted in improved growth at most recent follow-up among a diverse group of cardiac medical patients when compared with historical controls. Improved growth was observed despite the fact that SCAMP enrolled patients had less follow-up time from baseline to most recent weight measurement to achieve gains in weight-for-age z-score compared with controls. SCAMP iteration II patients showed greater positive change in weight-for-age z-score compared with all groups (Fig 2). As evidenced by this faster change in weight-for-age z-score, the effects of this SCAMP may be an initial push towards a positive growth trajectory for enrolled patients.
Our results are consistent with other data showing that standardisation of nutritional practices through bundles or practice algorithms with early involvement of dietitians can improve nutrition and growth outcomes. In one paediatric cardiac ICU, integration of nutrition goals into bedside rounds, mandatory involvement of feeding specialists, and introduction of an enteral nutrition guideline were associated with improved nutrition delivery and significant improvements in weight gain and nutritional status at the time of discharge.Reference Kaufman, Vichayavilas and Rannie 11 For outpatients with complex CHD, data from the National Pediatric Cardiology Quality Improvement Collaborative have shown that early identification of growth failure along with close monitoring by a dietitian improves growth outcomes during the interstage period.Reference Anderson, Beekman and Kugler 12
To our knowledge, this is the first study on paediatric CHD patients to assess the impact of an inpatient standardised feeding and nutrition protocol beyond hospital discharge. Other studies have demonstrated change in weight and weight-for-age z-score with different inpatient feeding approaches, hospital courses, and home feeding practices, but did not measure the impact of a standardised inpatient approach beyond hospital discharge.Reference Burch, Gerstenberger and Ravishankar 13 – Reference Peres, Croti and Godoy 18 It is possible that early attention to nutrition during an inpatient admission may influence both the providers and the families to continue paying close attention to nutrition even after discharge. Our study adds to previous data showing that interdisciplinary nutrition support teams, comprising a gastroenterologist or other physicians with nutrition expertise, registered dietitians, and sometimes additional staff, can educate clinicians and collaborate with them to improve healthcare outcomes.Reference Newcombe and Fry-Bowers 19 , Reference Simsic, Carpenito and Kirchner 20 In critically ill neonates and children some, although not all, studies suggest that involvement of a multidisciplinary nutrition support team can reduce inappropriate parenteral nutrition use and increase enteral nutrition, and may improve growth outcomes, length of ICU stay, and ICU mortality.Reference Lambe, Hubert, Jouvet, Cosnes and Colomb 21 – Reference Gurgueira, Leite, Taddei and de Carvalho 24 Expertise provided by otolaryngologists and speech pathologists can ensure early identification and treatment of patients with impaired oral feeding skills. Data from National Pediatric Cardiology Quality Improvement Collaborative on children with single-ventricle heart defect following stage 1 palliation identified that vocal cord injury and a lower target caloric goal at discharge were both independent risk factors for poor growth during the interstage period.Reference Hill, Hehir and Bartz 15 The studies showing a benefit from implementation of a nutrition support team often had other accompanying interventions, including standardised nutrition practice algorithms and/or associated audits and feedback provided by the nutrition support team.
There were a number of notable limitations to our study. The number of patients enrolled in SCAMP iteration II with at least 6 months of follow-up was relatively small, thereby limiting our ability to distinguish characteristics associated with better growth. Detailed data were not collected on the specifics of feeding practices, either inpatient or at home, and thus we were unable to describe feeding practices that resulted in better growth. There were multiple diversions for clinical reasons and we were unable to determine whether these patients would have shown better growth had they been enrolled. Last, we cannot exclude alternative reasons that could have explained our findings, such as the possibility that SCAMP iteration II patients were less sick or had better procedural results than control patients.
In conclusion, infants enrolled in a feeding and nutrition SCAMP had a greater positive change in World Health Organization weight-for-age z-score at a median follow-up of 1.8 years when compared with a group of historical controls. High-risk infants with CHD and growth failure managed with a full nutrition assessment with nutrition goals, identification of key diagnoses that may affect feeding, and close communication with nutrition experts during a medical admission showed an improved growth trajectory beyond hospital discharge. This study highlights the need for continued effort to improve growth in infants with CHD and the great utility of structured management plans such as SCAMPs.
Acknowledgements
The authors thank Ellen McCusty of the Heart Center Information Services at Boston Children’s Hospital for data acquisition and support.
Financial Support
The authors gratefully acknowledge support from the Hinden Family Fund to support SCAMPs research at Boston Children’s Hospital.
Conflicts of Interest
K.J.J. has been assigned Intellectual Property for SCAMPS through Boston Children’s Hospital and may receive royalties in the future. Boston Children’s Hospital and the Department of Cardiology may also receive royalties.
Ethical Standards
The study was approved by the Boston Children’s Hospital Institutional Review Board (IRB-P00019697) with a waiver of consent.










