Case report
A newborn female was born at 39 3/7 weeks gestational age at an outside hospital to a 32-year-old G2P0 Hawaiian female with adequate prenatal care. The mother had no history of any chronic health conditions and the pregnancy was reportedly uncomplicated, with normal prenatal ultrasounds and laboratory results. Apgar scores were 7 and 8 at 1 and 5 minutes, respectively. Shortly following delivery, the patient developed significant tachypnoea and cyanosis with oxygen desaturations to the 50’s on room air. The patient was started on non-invasive continuouspositive airway pressure and an alprostadilinfusion. Initial echocardiogram was concerning for hypoplastic left heart syndrome-variant versus Shone’s complex,and the patient was transferred to the cardiothoracic ICU at our institution for further care.
On arrival, the patient remained on an alprostadil infusion at 0.1 mcg/kg/minute, with oxygen saturations persistently in the 50’s despite ongoing respiratory support with nasal continuous positive airway pressure with FiO2 100%. The patient was immediately intubated but remained significantly desaturated. Repeat echocardiogram was obtained (Fig 1), which revealed the absence of pulmonary veins entering the atrium and absence of dilated pulmonary veins or large vertical vein. There was all right to left shunting across an unrestrictive atrial communication, and there was a large patent ductus arteriosus with predominantly right to left flow. A trivial pulmonary vein confluence was seen posterior to the left atrium. The right ventricle was dilated and the left ventricle was apex forming but appeared underfilled. Given that discrete pulmonary veins or venous drainage could not be delineated by echocardiography, cardiac CT scan was emergently obtained. Cardiac CT revealed a trivial pulmonary venous confluence with small left and right veins posterior to the left atrium; however, no discrete connection to the heart was identified (Fig 2). Due to the near atretic nature of this patient’s pulmonary veins and common pulmonary vein and lack of identified collaterals, extensive multidisciplinary discussions were held between Cardiology, Cardiac Intensive Care, Radiology, and Cardiothoracic Surgery teams. Ultimately, it was determined that there was no viable operative repair, and the decision was made between the family and the medical team to proceed with withdrawal of care. The patient was transferred back to the cardiothoracic ICU, compassionately extubated, and died shortly thereafter. An autopsy was performed which confirmed the absence of any pulmonary venous connection to the left atrium. There was a trivial pulmonary venous confluence superior to the left atrium as noted on the echocardiogram and CT, with a trivial vein that appeared to drain from the confluence to the innominate vein. No dilated pulmonary veins were demonstrated by autopsy. On gross examination, the lungs had a diffusely nodular appearance and histological examination revealed findings consistent with cystic lymphangiectasia.
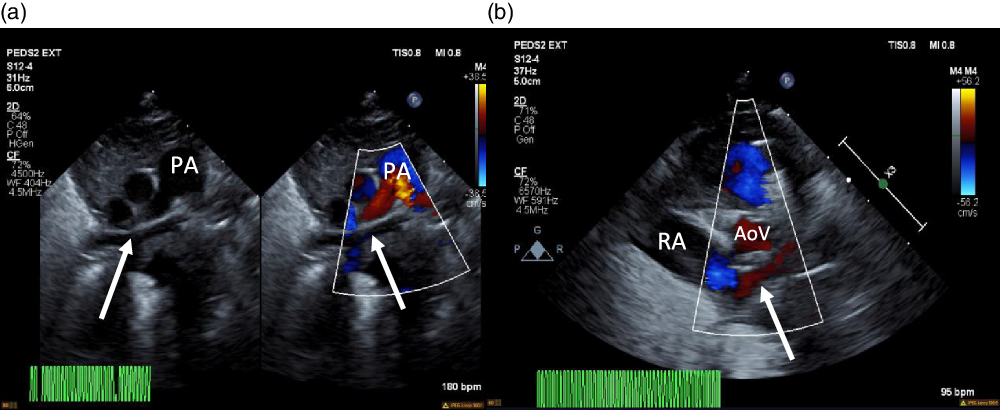
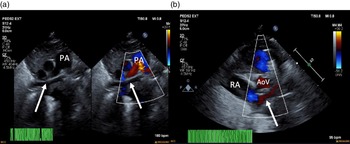
Figure 1. ( a ) 2D echocardiogram and colour echocardiography. Seen (arrows) is a trivial pulmonary venous confluence coursing superior to the left atrium (not seen). ( b ) Colour echocardiography. Underfilled left atrium (arrow) seen demonstrating the absence of pulmonary venous return to the left atrium; notably the right atrium is dilated. AoV = aortic valve; PA = pulmonary artery; RA = right atrium.
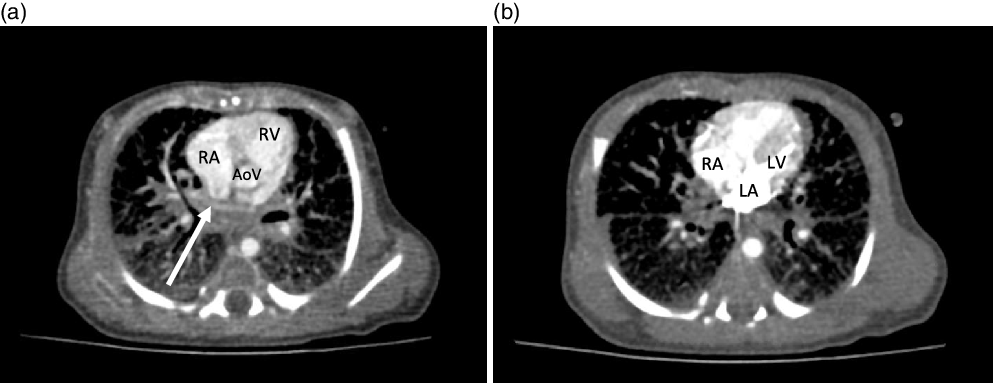
Figure 2. CT cardiac with contrast. ( a ) Axial view demonstrating the presence of an ill-defined opacified apparent pulmonary venous confluence superior to the left atrium (arrow). ( b ) Axial section demonstrating the absence of any pulmonary venous drainage to the left atrium during delayed phase injection. AoV = aortic valve; LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle.
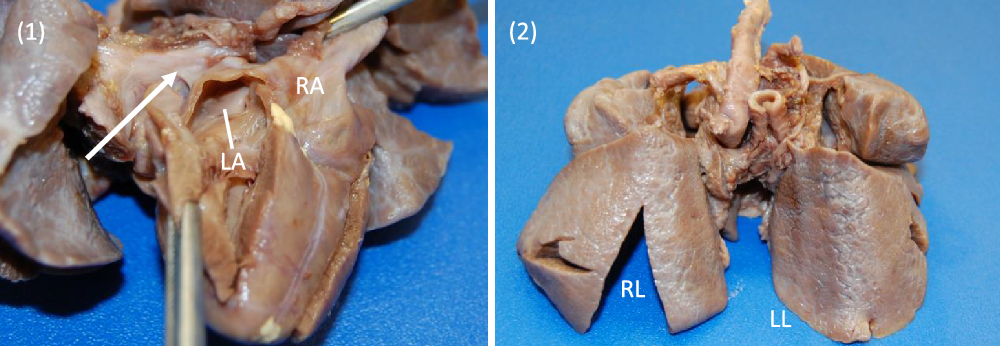
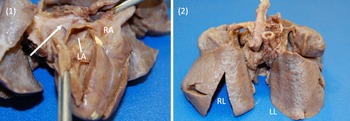
Images 1, 2. Gross specimen images. (1) Posterior view. A trivial pulmonary venous confluence is seen superior to the left atrium (arrow). The left atrium has been reflected open and there is no identifiablepulmonary venous drainage entering the left atrium. The right atrium is notably dilated. (2) Anterior view. The lungs have a diffusely nodular appearance consistent with the post-mortem microscopic findings of cystic lymphangiectasia.
Discussion
Common pulmonary vein atresia is a rare, but serious, form of total anomalous pulmonary venous return, which can lead to cyanosis and respiratory distress in the early postnatal period. As Lucas et al Reference Lucas, Woolfrey and Anderson1 first described in 1962, the common pulmonary vein is an embryonic structure that develops as an outgrowth of the heart that makes connection to the venous plexus which drains the pulmonary primordia. As development continues, the common pulmonary vein is eventually incorporated into the left atrial wall. The group suggests that in total anomalous pulmonary venous return with anomalous drainage, this connection between the left atrium and common pulmonary vein is obliterated early in development, such that collateral venous drainage develops and anomalous venous return persists. In cases of common pulmonary vein atresia, the common vein is thought to obliterate late in development, leading to the inability of collaterals to form and as a result, the presentation described above. Reference Lucas, Woolfrey and Anderson1 Various institutions have attempted to determine the exact incidence of this condition based on retrospective autopsy reviews, with reported results varying from 0.03 Reference Vaideeswar, Tullu and Sathe2 to 0.22% Reference Deshpande and Kinare3 of patients with suspected CHD. In 2008, Vaideeswar et al Reference Vaideeswar, Tullu and Sathe2 reported that the total number of documented cases of common pulmonary vein atresia in the literature to be around 25, a number which had grown to 35 by 2015, as reported by Perez et al. Reference Perez, Susheel Kumar and Briceno-Medina4 This number likely underrepresents the number of affected patients, given the difficulties associated with accurately establishing this diagnosis.
Cases of common pulmonary vein atresia present a unique surgical challenge, which is made more difficult because of the lack of prenatal diagnosis. In some instances, prenatal diagnosis of total anomalous pulmonary venous return is incorrectly made, and only after postnatal echocardiogram is performed, the diagnosis of common pulmonary vein atresia is made. Reference Nakamura5 Unfortunately, even with prompt diagnosis, survival is suboptimal even if operative repair is attempted and commonly depends on the presence of a sizeable venous confluence, Reference Perez, Susheel Kumar and Briceno-Medina4 which the patient in our case was lacking. In 2015, Perez et al described a series of three patients with similar anatomic findings to the patient in our institution, none of whom were offered surgical repair. Reference Perez, Susheel Kumar and Briceno-Medina4 Of the less than 40 reported cases of common pulmonary vein atresia, there have been only 5 successful surgical repairs, Reference Dudell, Evans and Krous6-Reference Suzuki, Sato and Murai8 highlighting the typical poor prognosis. Additionally, long-term data on these few survivors are not available.
Unfortunately, without surgical repair, common pulmonary vein atresia is universally fatal. Our report of a patient with common pulmonary vein atresia demonstrates the need for prompt diagnosis and evaluation by echocardiography, CT, and/or cardiac catheterisation to determine the presence and/or size of a pulmonary venous confluence. It also emphasises the importance of a cohesive, multidisciplinary approach between multiple subspecialty teams in evaluating and determining surgical candidacy and best course of action. As more patients with common pulmonary vein atresia are identified, hopefully necessary, continued reporting and expanded,ongoing research into this rare population will continue.
Acknowledgements
The authors would like to acknowledge the cardiothoracic surgical team at our institution for their contributions to medical discussions about this case.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflicts of interest
None.